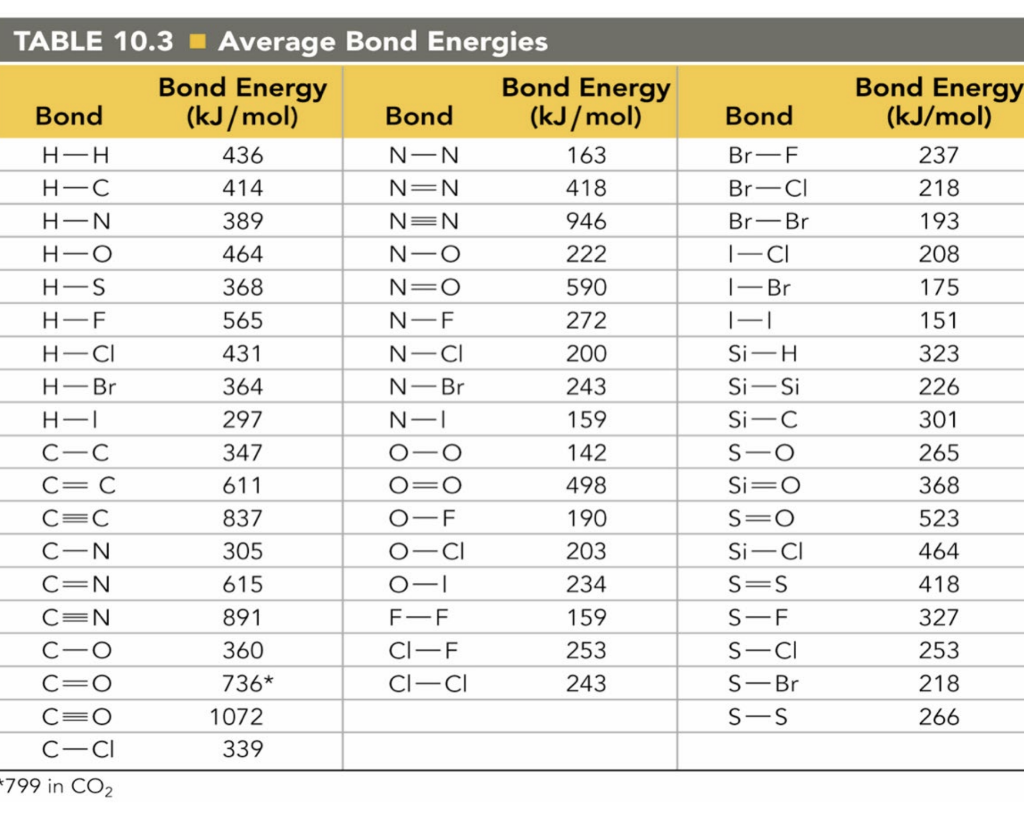
Bond Energies Chart
Bond Energies Chart - Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively. You can take all these terms as meaning the same thing. You can calculate the energy change in a reaction using average bond energies. Typical units are kilocalories per mole (kcal/mol) or kilojoules per mole (kj/mol). The following tables list experimental bond dissociation enthalpies of common bonds at 298 k. The exact bond enthalpy of a particular chemical bond depends upon the molecular environment in which the bond exists. Web quantifying the energy represented by the bonds in different molecules is an important part of understanding the overall energy implications of a reaction. We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to. Bond energies are also called bond enthalpies, and in the past have been known as bond strengths. Bond length and bond energy. Web many of the bond energies listed here were taken from the following sources: Knowing the energies of bonds present in products and substrates, we can estimate the enthalpy of the chemical reaction: Web properties of atoms, radicals, and bonds 4.41 table 4.11 bond dissociation energies the bond dissociation energy (enthalpy change) for a bond a 9b which is broken. The si units used to describe bond energy is kilojoules per mole of bonds (kj/mol). Web properties of atoms, radicals, and bonds 4.41 table 4.11 bond dissociation energies the bond dissociation energy (enthalpy change) for a bond a 9b which is broken through the reaction ab : Energy is released when chemical bonds are formed because atoms become more stable.. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. Values listed in tables of bond energy and bond length are averages taken over a variety of compounds that contain a specific atom pair. The si units used to describe bond energy is kilojoules per mole of bonds. Cottrell, the strengths of chemical bonds, 2nd ed., butterworths, london, 1958; Web quantifying the energy represented by the bonds in different molecules is an important part of understanding the overall energy implications of a reaction. In this article, we'll explore two different concepts that help describe that energy: You can take all these terms as meaning the same thing. In. Mean bond enthalpies are sometimes referred to as bond enthalpy terms. Interpreting potential energy curves of diatomic molecules. Values listed in tables of bond energy and bond length are averages taken over a variety of compounds that contain a specific atom pair. Web many of the bond energies listed here were taken from the following sources: Bond energy is the. That is, hf 298 298 298 298 hf (a). Knowing the energies of bonds present in products and substrates, we can estimate the enthalpy of the chemical reaction: The si units used to describe bond energy is kilojoules per mole of bonds (kj/mol). Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential. Energy is required to break bonds. Mean bond enthalpies are sometimes referred to as bond enthalpy terms. Web bond energy (be) is the average amount of energy required to break all the chemical bonds between the same two types of atoms in a molecule (e.g., carbon and hydrogen, hydrogen and oxygen). Bond order is the number of electron pairs that. Energy is released when chemical bonds are formed because atoms become more stable. We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to. Web the average bond energy is therefore +1662/4 kj, which is +415.5 kj per mole of bonds. Intramolecular force and potential energy get. Web bond energy (be) is the average amount of energy required to break all the chemical bonds between the same two types of atoms in a molecule (e.g., carbon and hydrogen, hydrogen and oxygen). Values listed in tables of bond energy and bond length are averages taken over a variety of compounds that contain a specific atom pair. Web the. Web quantifying the energy represented by the bonds in different molecules is an important part of understanding the overall energy implications of a reaction. This distance is the bond length between the atoms. Values listed in tables of bond energy and bond length are averages taken over a variety of compounds that contain a specific atom pair. You can take. R.t.sanderson, polar covalence , 1983 r.t.sanderson, chemical bonds and bond energy , 1976 Values listed in tables of bond energy and bond length are averages taken over a variety of compounds that contain a specific atom pair. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). Web the average bond energy is therefore +1662/4 kj, which is +415.5 kj per mole of bonds. That is, hf 298 298 298 298 hf (a). The following tables list experimental bond dissociation enthalpies of common bonds at 298 k. Web bond energy (be) is the average amount of energy required to break all the chemical bonds between the same two types of atoms in a molecule (e.g., carbon and hydrogen, hydrogen and oxygen). Knowing the energies of bonds present in products and substrates, we can estimate the enthalpy of the chemical reaction: Energy is released when chemical bonds are formed because atoms become more stable. It is also called average bond enthalpy or mean bond enthalpy. Web atoms are held together by a certain amount of energy called bond energy. Web quantifying the energy represented by the bonds in different molecules is an important part of understanding the overall energy implications of a reaction. Web the energy of chemical bonding is given most often in kilojoules per mole : Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively. In proposing his theory that octets can be completed by two atoms sharing electron pairs, lewis provided scientists with the first description of covalent bonding. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases.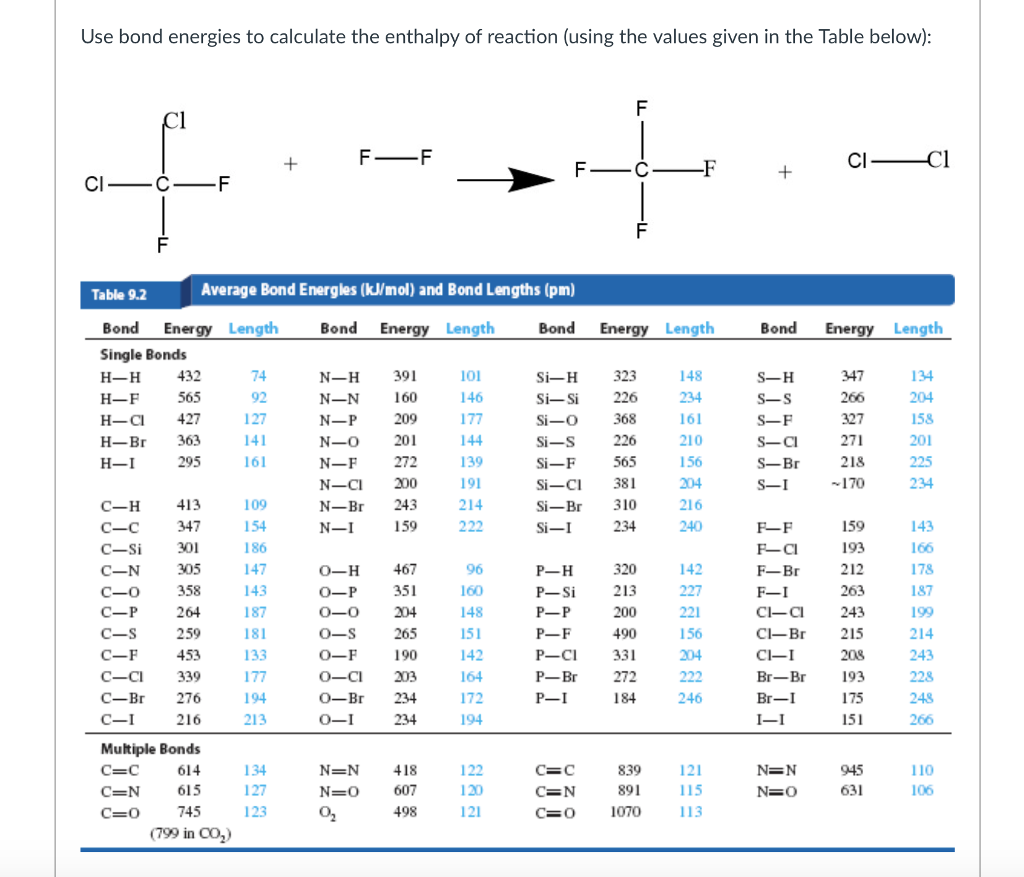
Bond Energies Chart
Solved Bond TABLE 10.3 Average Bond Energies Bond Energy

Bond Energy and Strength
[Solved] Using the appropriate bond energies, calculate the heat of

Average Bond Energies Chart
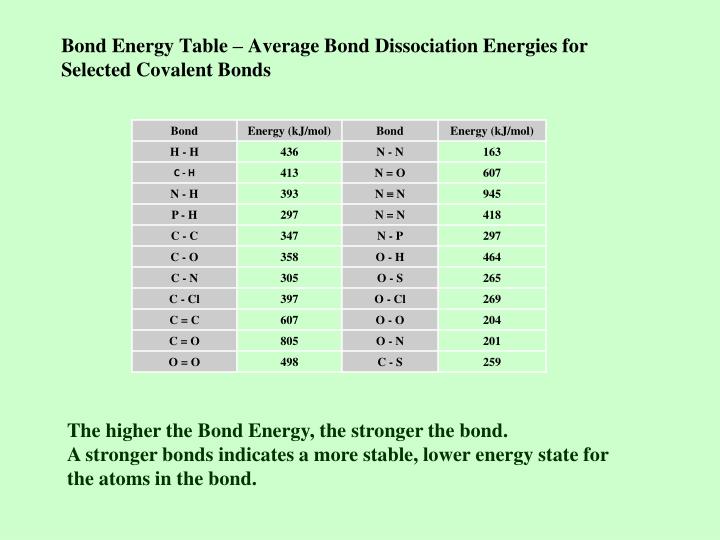
Bond Energy Profile Chart
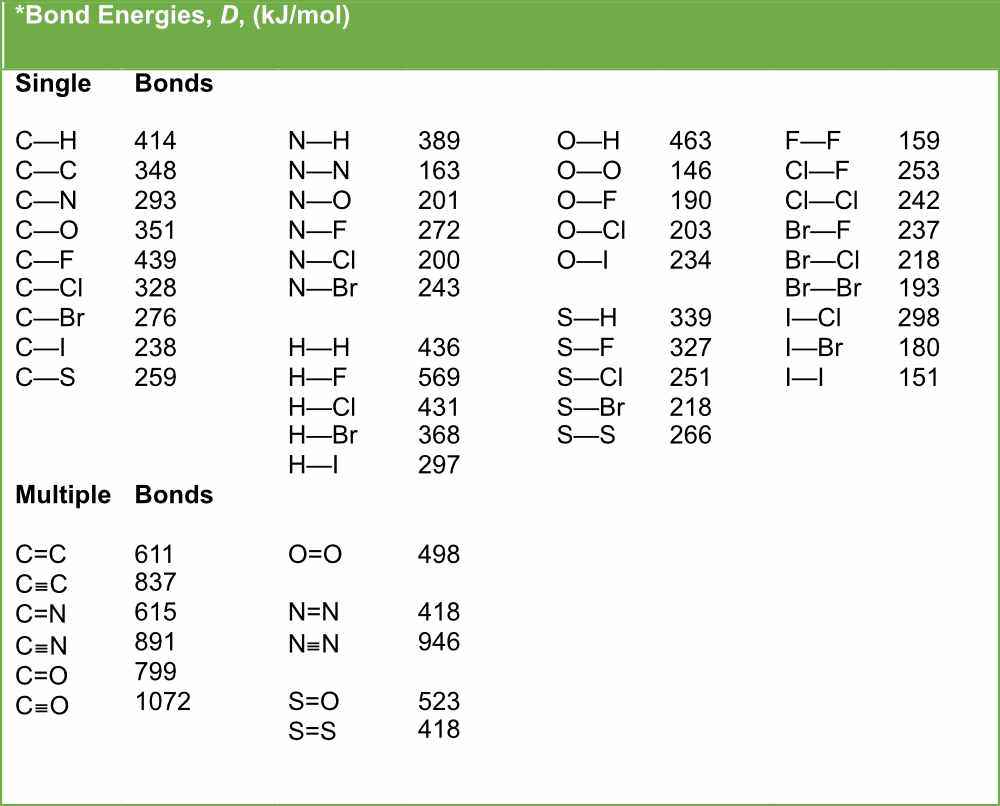
Table of Bond Energies Pathways to Chemistry
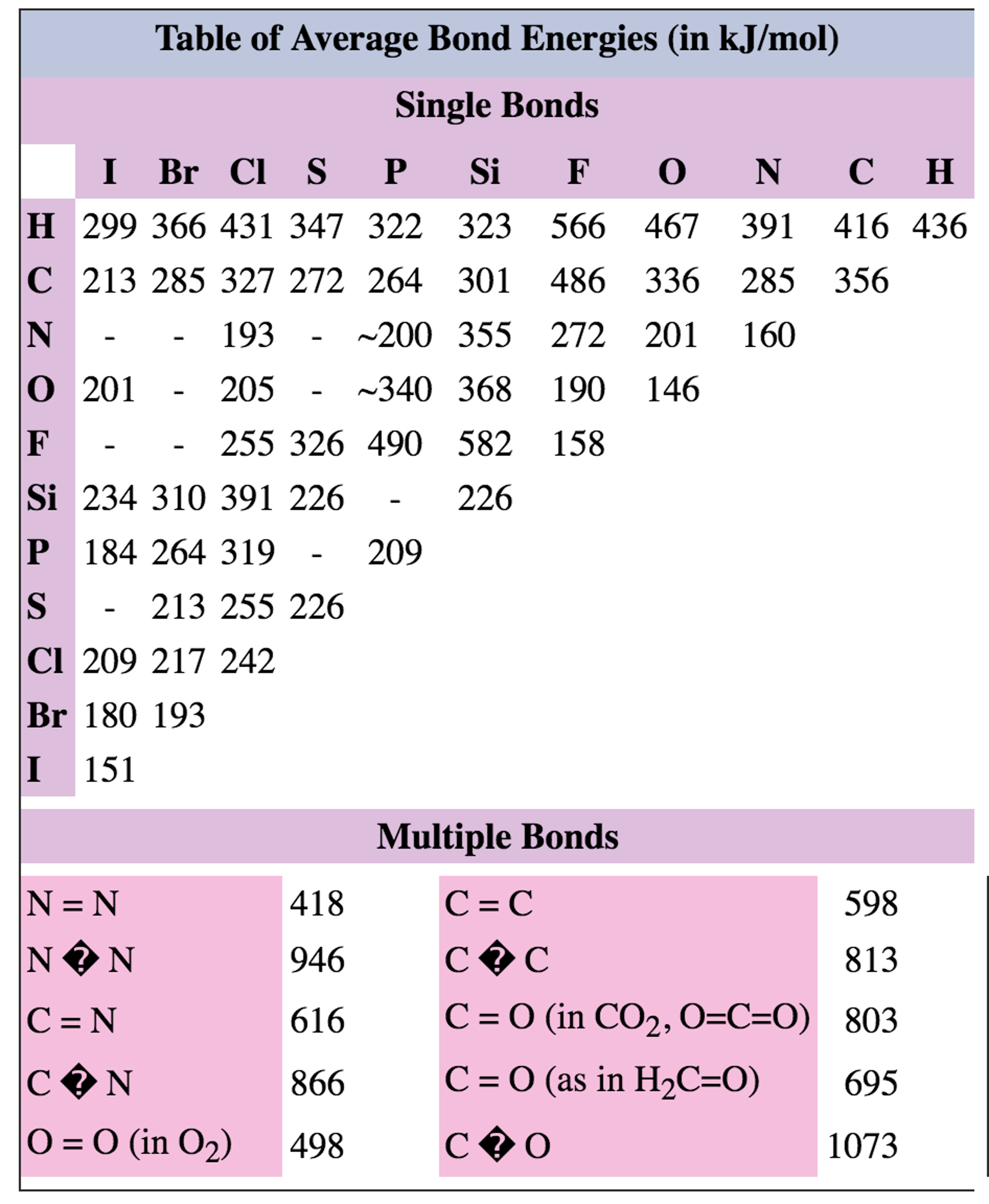
Bond Energies Chart
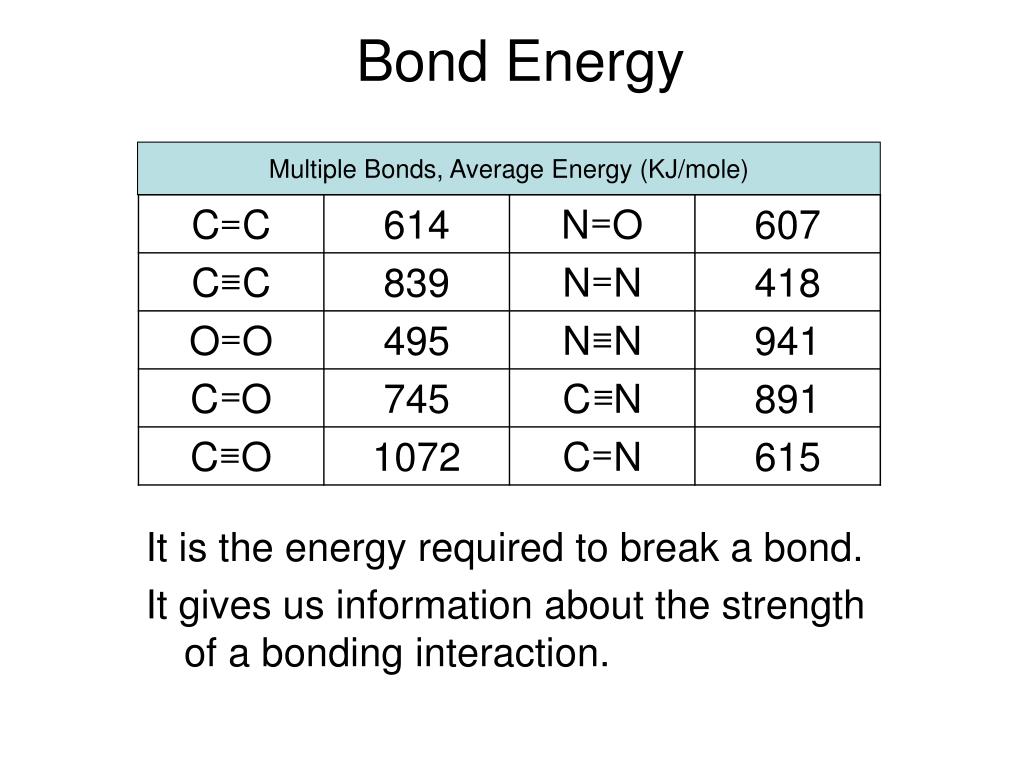
PPT Covalent Bonding PowerPoint Presentation, free download ID2110028
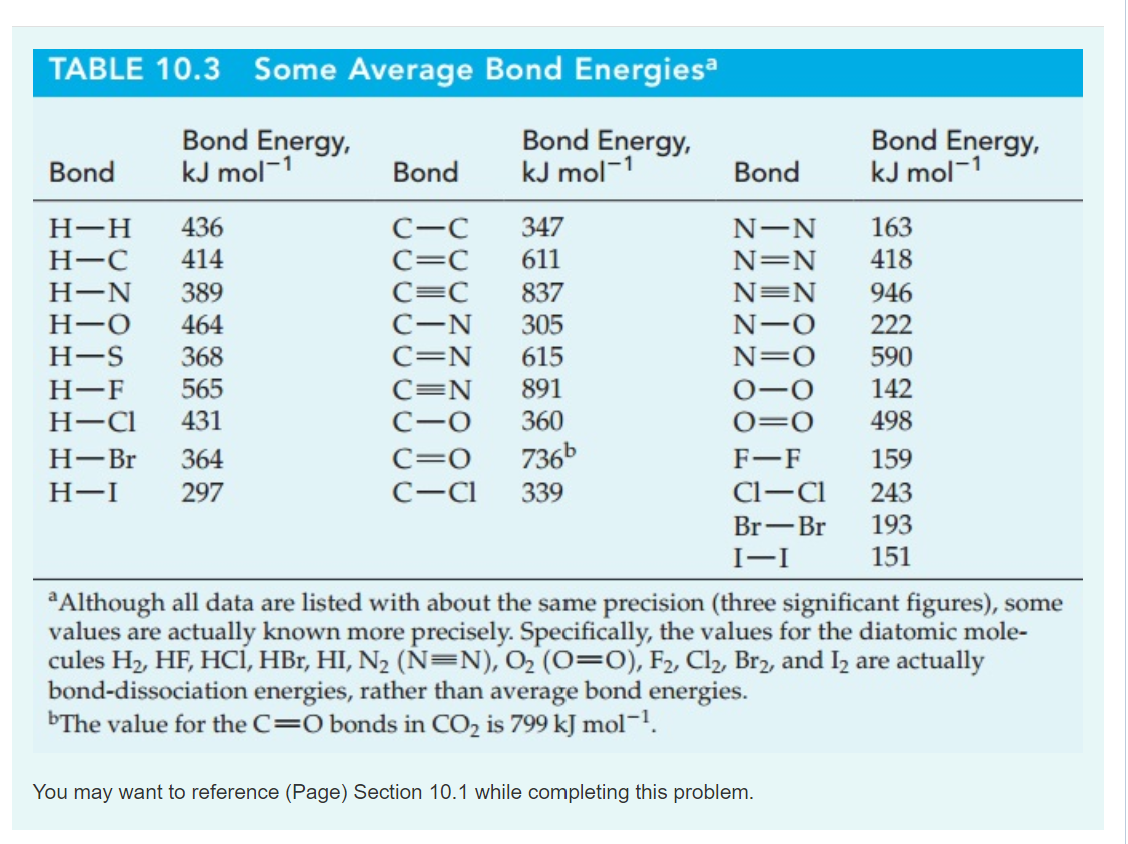
Table Of Bond Energies Pathways To Chemistry vrogue.co
Mean Bond Enthalpies Are Sometimes Referred To As Bond Enthalpy Terms.
This Distance Is The Bond Length Between The Atoms.
In This Article, We'll Explore Two Different Concepts That Help Describe That Energy:
Energy Is Required To Break Bonds.
Related Post: