Bond Length Chart
Bond Length Chart - A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). Lewis diagram of formaldehyde (ch₂o) worked example: Web bond length is defined as the distance between the centers of two covalently bonded atoms. I know this is a late response, but from what i gather we can tell what the bond order is by looking at the number of valence electrons and how many electrons the atoms need to share to complete their outer shell. Darwent, national standard reference data series, national bureau of standards, no. Web below is a table of average bond lengths. Bond order and length are inversely proportional to each other: Web bond order and bond length indicate the type and strength of covalent bonds between atoms. Bond length & bond strength. Bond energy is the potential energy required to break a covalent bond. Bond length to change units) The bond length is internuclear distance of two covalently bonded atoms. Lewis diagram of xenon difluoride (xef₂) practice. Bond order and length are inversely proportional to each other: Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond). In real world, distance beetwen atoms are not constant, because atoms in mocelules are in constant motion. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum). A shorter bond length has higher bond energy. The bond length is internuclear distance of two covalently bonded atoms. Web bond length is defined as the distance between the centers of two covalently bonded atoms. Web ( 10 votes) upvote. Predicting bond type (metals vs. Web in molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. Bond length & bond strength. Web the bond length between any two adjacent nuclei in such a covalent molecule is the distance between the two nuclei at the minimum energy in a graph of energy versus. Web in molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length. Bond length & bond strength. Bond length and bond strength. Single, double, or triple bond). Single, double, or triple bond). When bond order is increased, bond length is decreased. The bond length is internuclear distance of two covalently bonded atoms. For example, the bond length of h2 is approximately 62pm, since both atoms are hydrogen atoms and their covalent radius is 31pm. As we go across a period we see bond length decreases. Bond length is the average distance between the two nuclei of atoms bonded together in a covalent bond. For example, the bond length of h2 is approximately 62pm, since both atoms are hydrogen atoms and their covalent radius is 31pm. This section further explores bond length and bond strength, which were introduced in the previous section. Cottrell, the strengths of. A shorter bond length has higher bond energy. Web common bond energies (d. Predicting bond type (metals vs. Bond length and bond energy. For example, the bond length of h2 is approximately 62pm, since both atoms are hydrogen atoms and their covalent radius is 31pm. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Predicting bond type (metals vs. The bond length is internuclear distance of two covalently bonded atoms. Web silans # some facts # when we say about bond length in the molecule we get in mind the distance between atomic nuclei. Web to calculate bond length, one must draw. This section further explores bond length and bond strength, which were introduced in the previous section. The bond energy is the energy required to break one mole of a particular covalent bond in the gaseous states. Web there are charts that reveal the estimated bond length (radii in angstroms or picometers) between two given atoms, according to bond type (i.e.. This is because as we go down the group, the atomic radius increases. Bond order is the number of electron pairs that hold two atoms together. The bond length in a h 2 molecule is 74 pm. Bond order and length are inversely proportional to each other: The bond length is internuclear distance of two covalently bonded atoms. Web bond order and bond length indicate the type and strength of covalent bonds between atoms. Test your knowledge with multiple choice flashcards. The bond energy is inversely proportional to the bond length. Web silans # some facts # when we say about bond length in the molecule we get in mind the distance between atomic nuclei. Web the bond length between any two adjacent nuclei in such a covalent molecule is the distance between the two nuclei at the minimum energy in a graph of energy versus nuclear separation. Covalent bonds can be characterized on the basis of several bond parameters such as bond length, bond angle, bond order, and. Web there are charts that reveal the estimated bond length (radii in angstroms or picometers) between two given atoms, according to bond type (i.e. Web below is a table of average bond lengths. Bond length and bond strength. Web bond length is defined as the distance between the centers of two covalently bonded atoms. In proposing his theory that octets can be completed by two atoms sharing electron pairs, lewis provided scientists with the first description of covalent bonding.
Average Bond Enthalpy Table
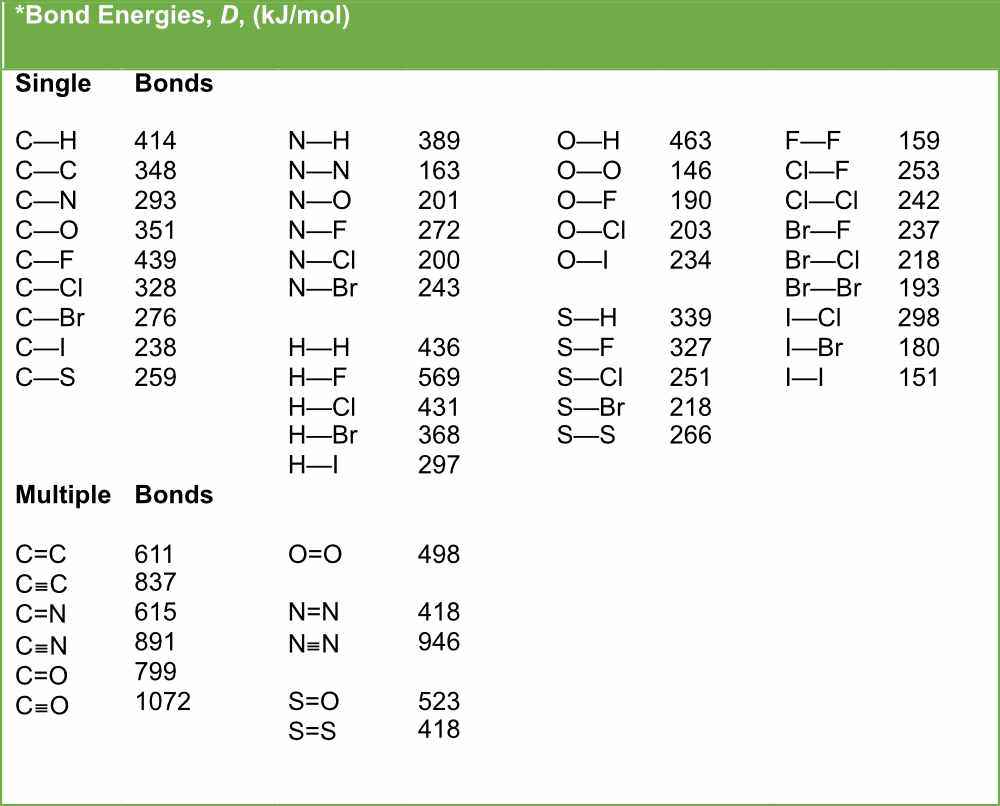
Bond Length and Bond Strength Pathways to Chemistry
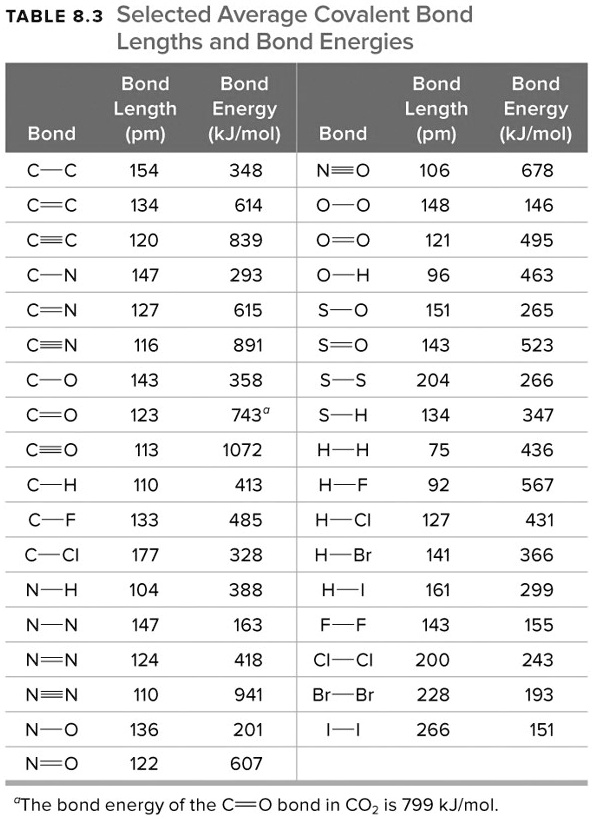
SOLVEDTABLE 8.3 Selected Average Covalent Bond Lengths and Bond

Individual bondstrengthbondlength parameters for MO bonds in the

Bond Length and Bond Strength Pathways to Chemistry

PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint

Bond Length Periodic Table

Selected bond lengths (Å), bond angles ( • ), and intermolecular

Bond Length and Bond Strength Chemistry Steps
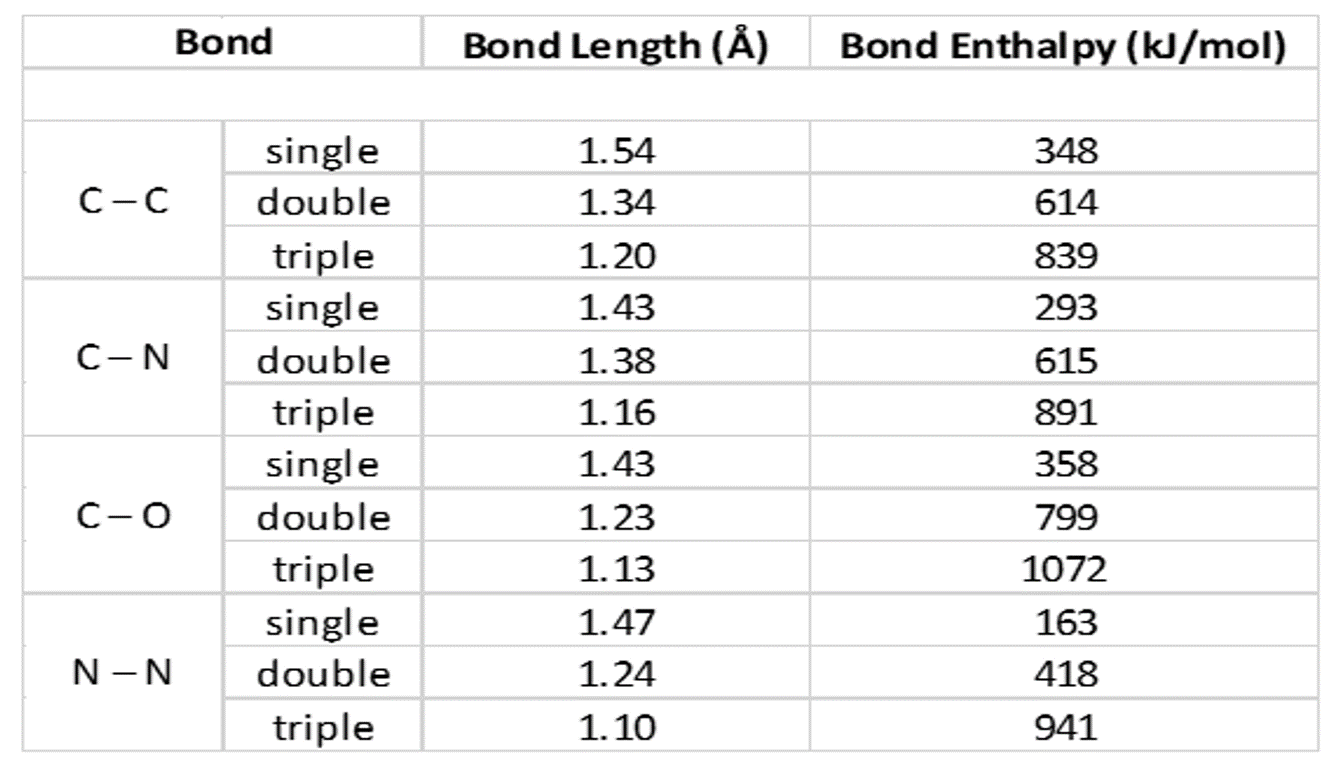
The bond lengths of carboncarbon, carbonnitrogen, carbono Quizlet
Lewis Diagram Of The Cyanide Ion (Cn⁻) Exceptions To The Octet Rule.
Single Bonds Have A Bond Order Of One, And Multiple Bonds With Bond Orders Of Two (A Double Bond) And Three (A Triple Bond).
For Example, The Bond Length Of H2 Is Approximately 62Pm, Since Both Atoms Are Hydrogen Atoms And Their Covalent Radius Is 31Pm.
Darwent, National Standard Reference Data Series, National Bureau Of Standards, No.
Related Post: