Chart That Compares Acids And Bases
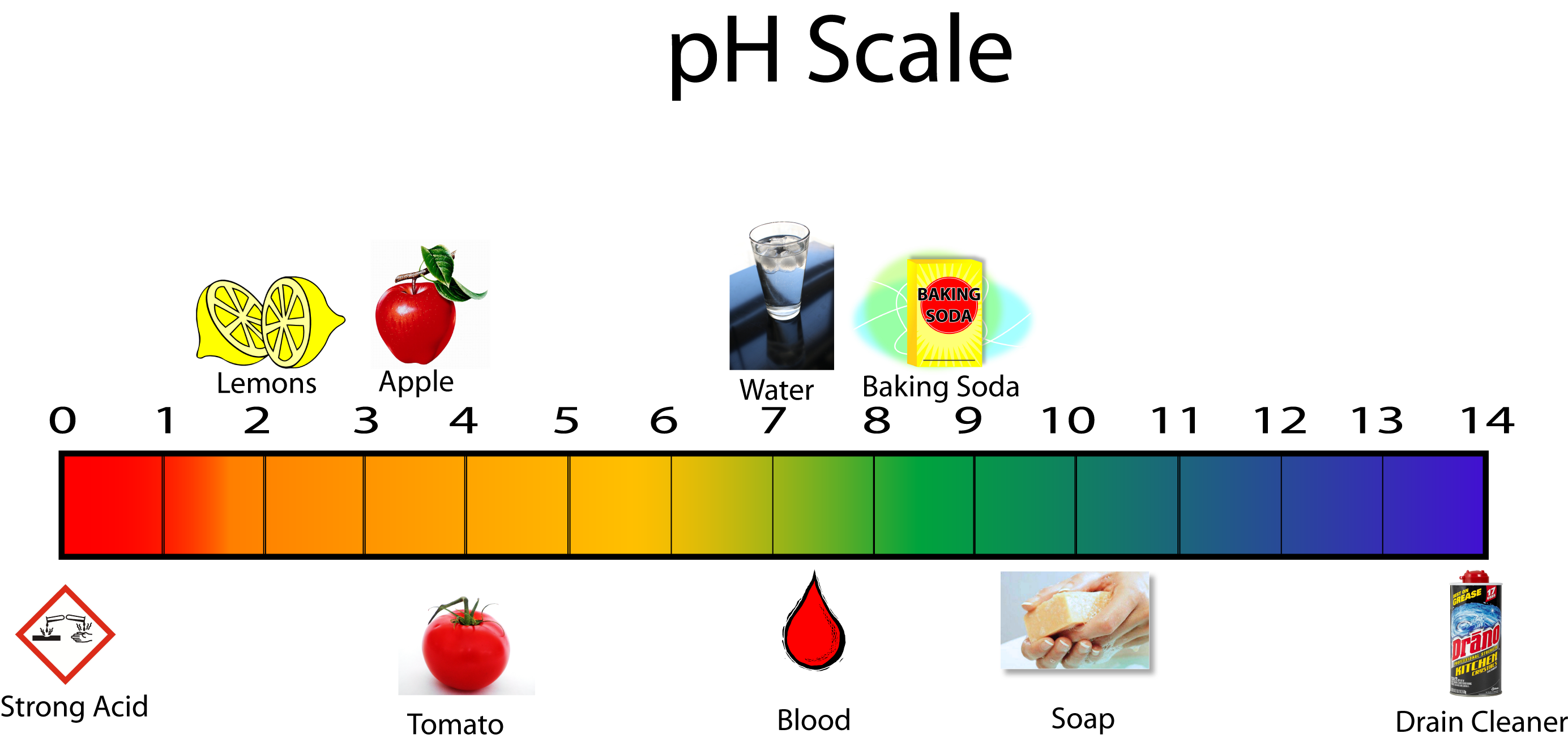
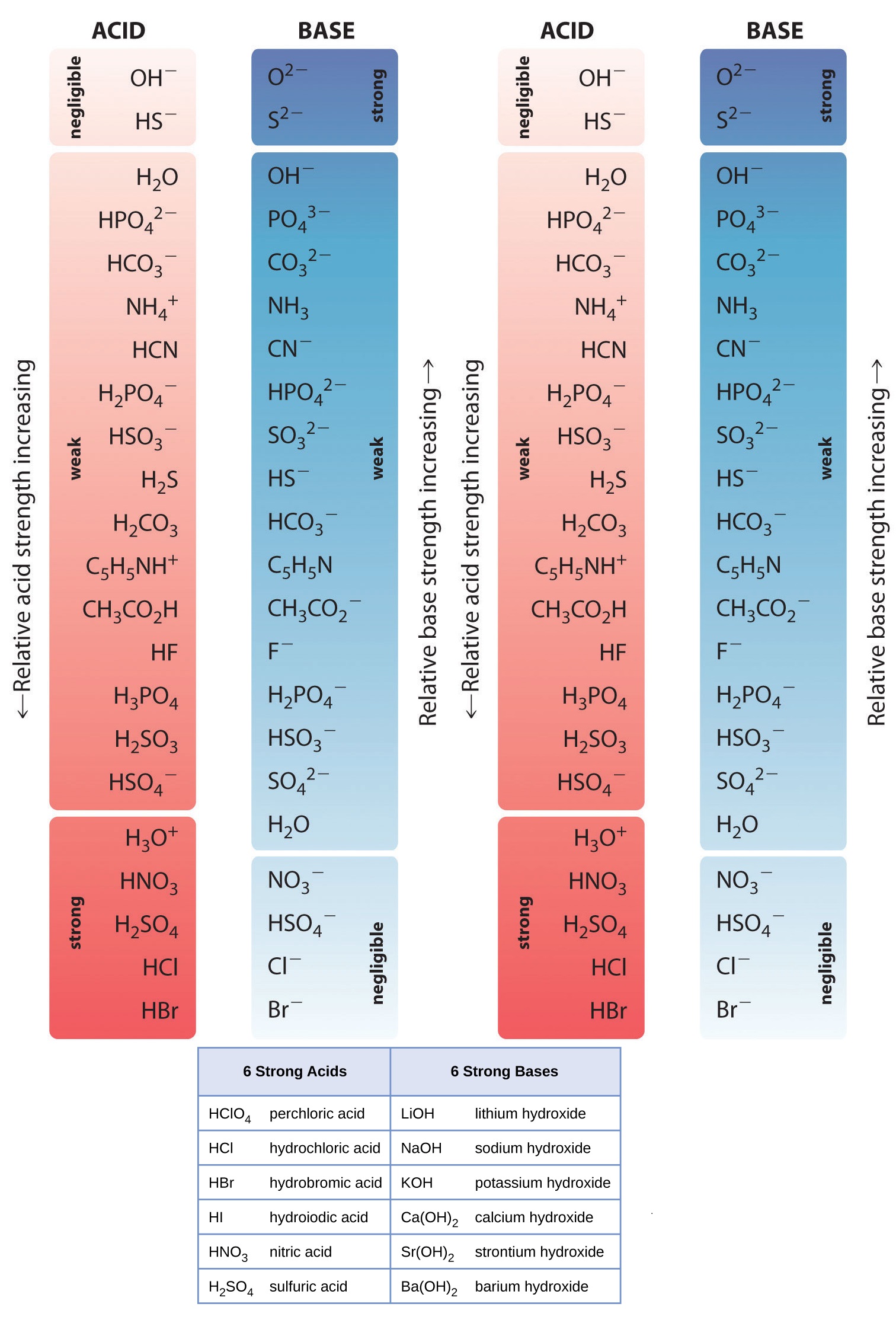
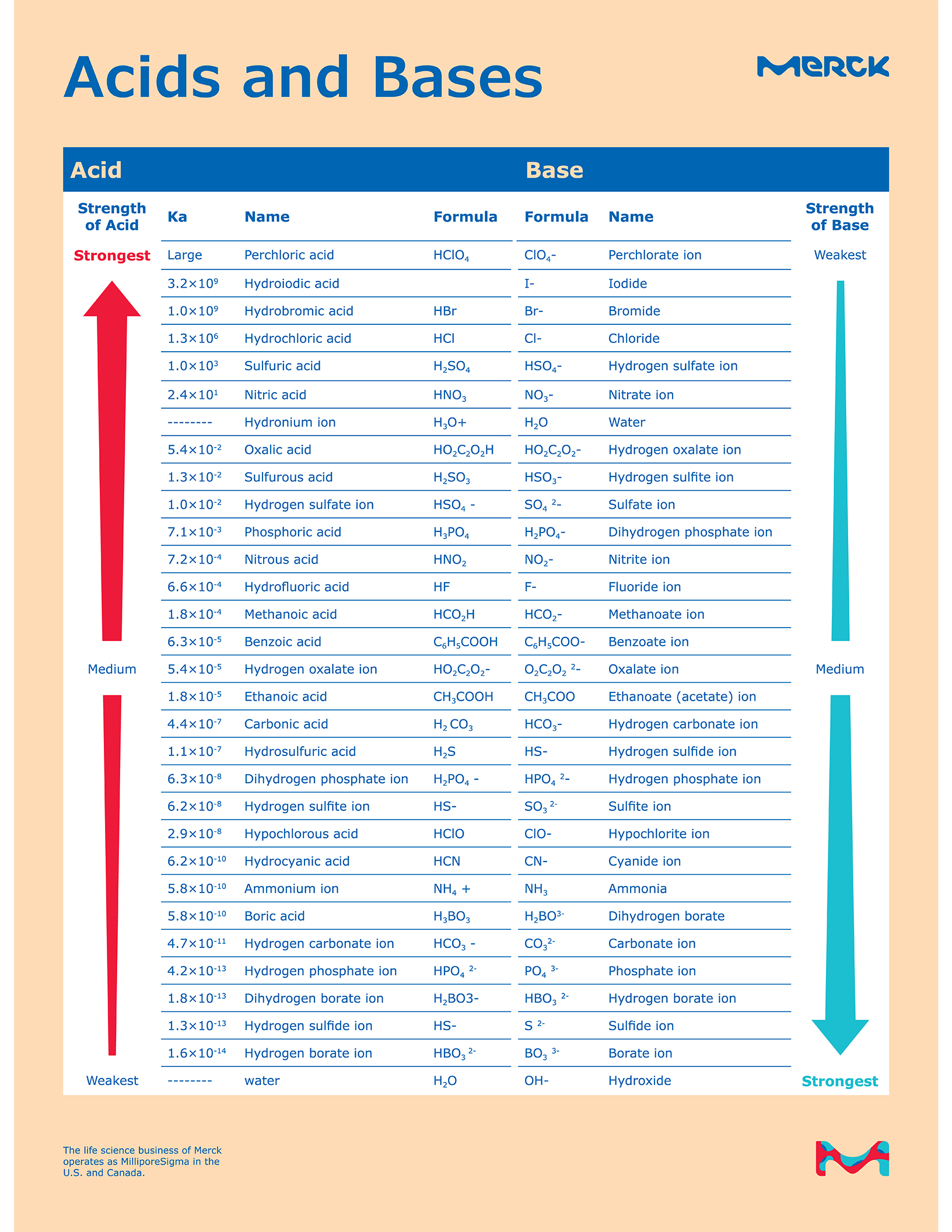
Chart That Compares Acids And Bases - One way of classifying acids and bases is as strong or weak: Web use this acids and bases chart to find the relative strength of the most common acids and bases. Ph values of common chemicals. What is acid in chemistry? Web chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. Web the earliest definition of acids and bases is arrhenius's definition which states that: What are acids and bases? Web there are three major classifications of substances known as acids or bases. Web properties of acids and bases. Acids and bases are distinct classes of compounds because of the properties of their aqueous solutions. Web there are three major classifications of substances known as acids or bases. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Web in chemistry, acids and bases have been defined differently by three sets of theories: Essentially, acids accept electron pairs and donate hydrogen protons. So what is an acid? Web understand the difference between acid and base, compare and contrast acids and bases. You’ll see charts that give slightly different ph values for some chemicals. So what is an acid? Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. One way of classifying acids and bases is as strong or weak: Web acids are sour, while bases are bitter. Acidic solutions have a higher h + concentration than water (greater than 1 × 10 − 7 m), while basic (alkaline) solutions have a lower h + concentration (less than 1 × 10 − 7 m). Web to understand the differences between the three definitions of acids and bases. 1 provides a. These dissociate completely in water. What are acids and bases? So what is an acid? Simple to use laboratory reference chart for scientists, researchers and lab technicians. Acids taste sour and create a stinging feeling on the mucous membranes. So what is an acid? Web study with quizlet and memorize flashcards containing terms like acid differences, base differences, similarities and more. Simple to use laboratory reference chart for scientists, researchers and lab technicians. Web acids are sour, while bases are bitter. Essentially, acids accept electron pairs and donate hydrogen protons. Web the earliest definition of acids and bases is arrhenius's definition which states that: Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Essentially, acids accept electron pairs and donate hydrogen protons. These dissociate completely in water. Web study with quizlet and memorize flashcards containing terms like acid differences, base differences, similarities. In this chapter, we will examine the properties of acids and bases, and learn about the chemical nature of these important compounds. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Web understand the difference between acid and base, compare and contrast acids and bases. Web acids are usually h+ donors. They change litmus paper red. Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. Strength of acids and bases. Simple to use laboratory reference chart for scientists, researchers and lab technicians. Examples include hydrochloric acid (hcl) and sodium hydroxide (naoh). Acids and bases are distinct classes of compounds because of the properties of their aqueous solutions. Web there are three major classifications of substances known as acids or bases. Web acids are sour, while bases are bitter. Web the earliest definition of acids and bases is arrhenius's definition which states that: This theory was developed by. This theory was developed by. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Web acids and bases can be described using the arrhenius model: Web properties of acids and bases. Examples include hydrochloric acid (hcl) and sodium hydroxide (naoh). Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. Acidic substances are usually identified by their sour taste. Discover common acids and bases examples in everyday household appliances. This theory was developed by. Simple to use laboratory reference chart for scientists, researchers and lab technicians. Examples include hydrochloric acid (hcl) and sodium hydroxide (naoh). Difference between acid and base. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. What is acid in chemistry? To give you a more clear understanding of the differences between acids and bases here is a comparison chart that you can refer to. Acids and bases are distinct classes of compounds because of the properties of their aqueous solutions. Here is a table of ph values of common chemicals. They can react with bases to produce salts and water. Web study with quizlet and memorize flashcards containing terms like how do polar molecules form *hydrogen bonds*?, what determines whether a compound will dissolve in water?, make a chart that compares *acids* and *bases.* and more. What are acids and bases? Web chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases.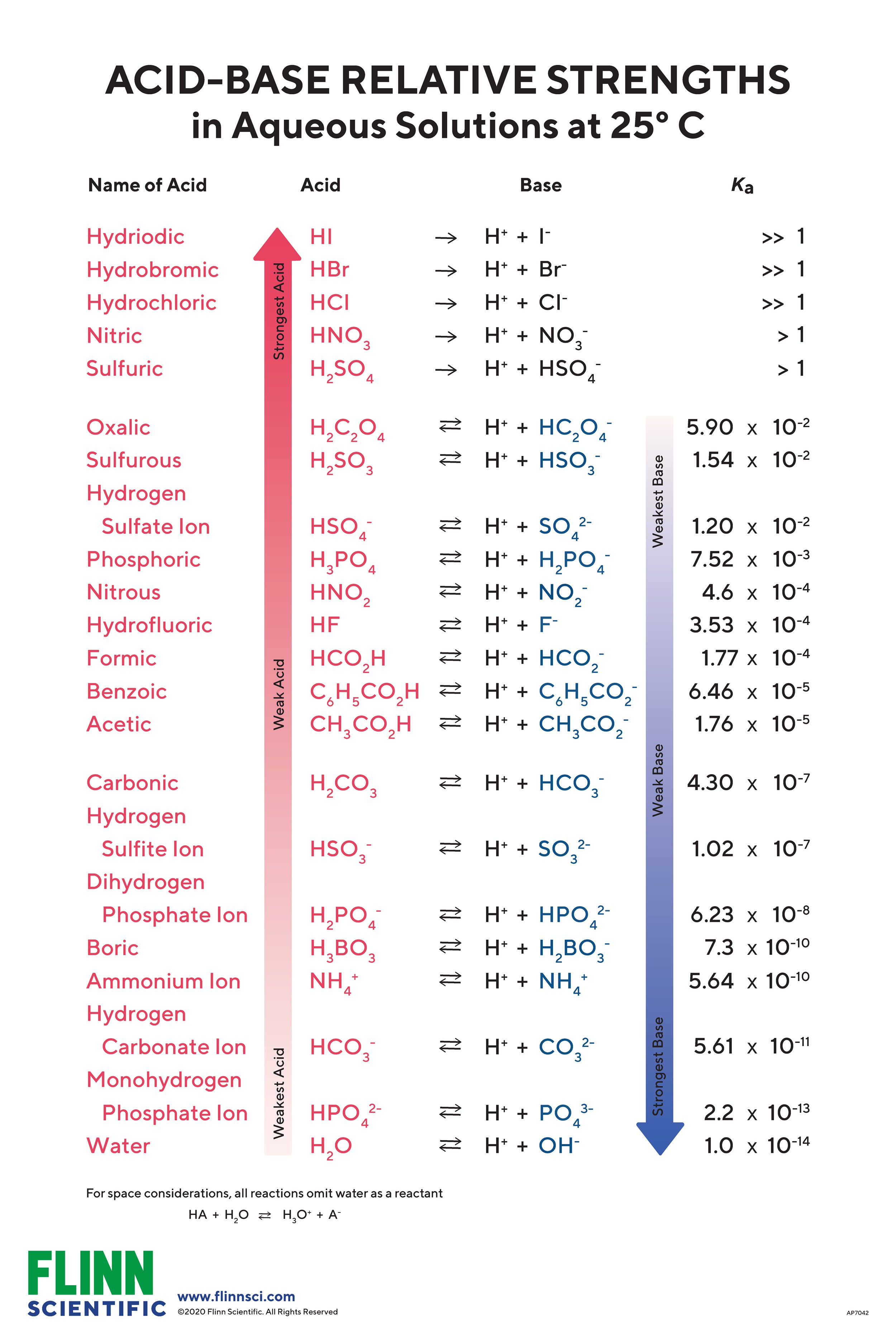
AcidBase Strength Charts for Chemistry
Acids and Bases MCHS Science
List of Strong Acids & Bases in Order StudyPK
Ph Table Chart
Acid and Base Chart — Table of Acids & Bases

Ph Scale Acids And Bases

Acids & Bases Visual (Perfecta Template) Teaching chemistry
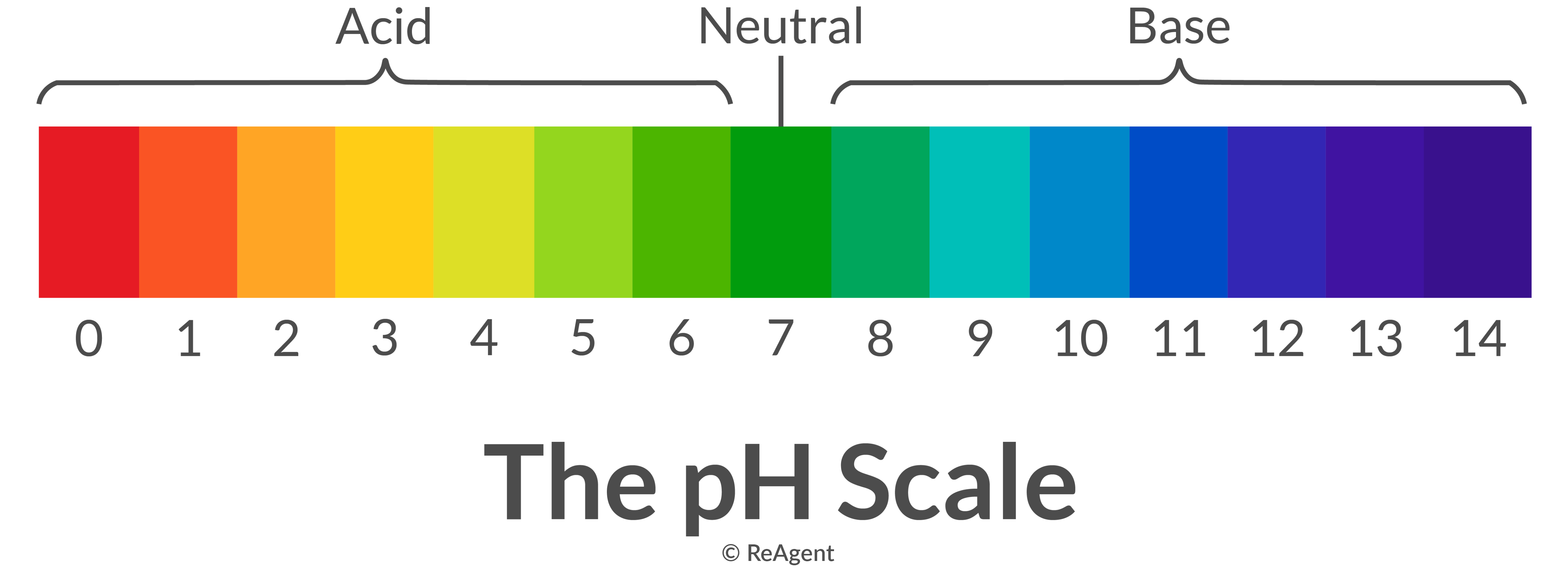
What’s The Reaction Between An Acid And A Base?
Acid And Base Venn Diagram Drivenheisenberg
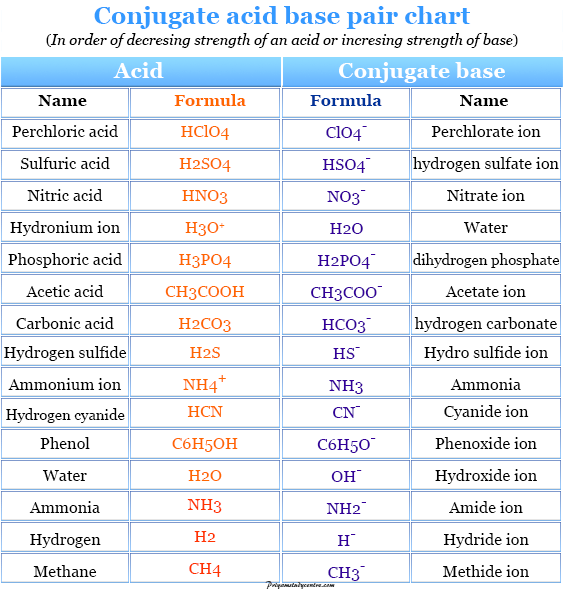
Acid And Base Chart
Simple To Use Laboratory Reference Chart For Scientists, Researchers And Lab Technicians.
Web To Understand The Differences Between The Three Definitions Of Acids And Bases.
1 Provides A Comparison Of Their Mostly Contrasting Properties.
Web Acid And Base Chart Lists The Strength Of Acids And Bases (Strongest To Weakest) In Order.
Related Post:
