Dissimilar Metals Corrosion Chart
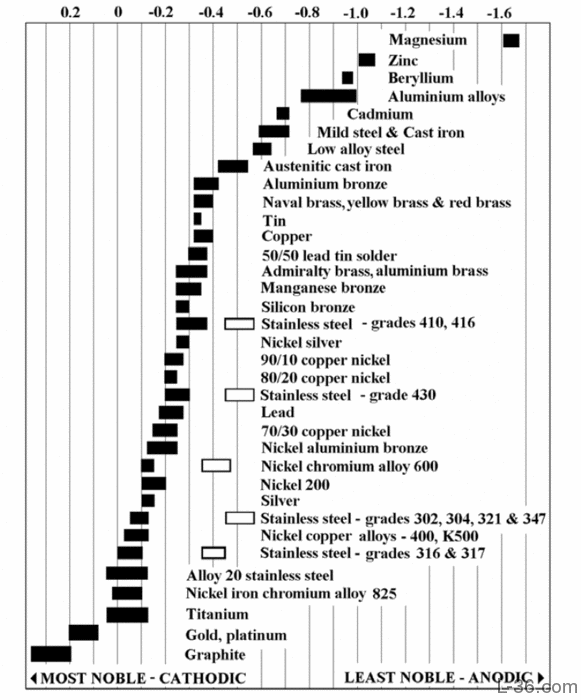
Dissimilar Metals Corrosion Chart - Dissimilar metals used in conjunction; Web the most important type of corrosion for understanding dissimilar metals is galvanic corrosion. Web below is a galvanic reaction chart for dissimilar metals. Contact a corrosion specialist to determine the best. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web galvanic/dissimilar metal or bimetallic corrosion is a type of electrochemical corrosion, where a material corrodes if it comes in contact with another material in the presence of. Web metals are rated in their ability to resist electrochemical corrosion on the scale of nobility and on the galvanic series chart, which shows their electrical potential in seawater. Metals close to one another on the chart. Web the common factors are dissimilar metals, electrical contact, and a conductive electrolyte in contact with them. So, for example, choosing zinc on zinc would have the lowest. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web the larger the separation distance in the electromotive chart between the two metals in contact, the higher the contact potential and chances for corrosion. Galvanic corrosion happens when dissimilar metals rub against one.. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Galvanic corrosion happens when dissimilar metals rub. This phenomenon is named after italian ph… Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. A typical rule of thumb is that voltage differences. Dissimilar metals used in conjunction; Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which. Metals close to one another on the chart. This phenomenon is named after italian ph… Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web below is a galvanic reaction chart for dissimilar metals. Galvanic corrosion happens when dissimilar metals rub against one. Web galvanic corrosion may be experienced when two dissimilar metals or alloys, not in direct contact, are nevertheless connected electrically. Web the larger the separation distance in the electromotive chart between the two metals in contact, the higher the contact potential and chances for corrosion. Web when two different metals are in contact and exposed to a common electrolyte, one. This phenomenon is named after italian ph… Obviously galvanic corrosion is more. Web the following three factors often contribute to a higher risk of galvanic corrosion: Web metals are rated in their ability to resist electrochemical corrosion on the scale of nobility and on the galvanic series chart, which shows their electrical potential in seawater. Web when two different metals. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). When dissimilar metals are used together in the presence of an electrolyte,. In the case of galvanic corrosion, the combination of two dissimilar metals with an electrolyte is all that is needed to form a. This phenomenon. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Web galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical. Web the most important type of corrosion for understanding dissimilar metals is galvanic corrosion. Web below is a galvanic reaction chart for dissimilar metals. In the case of galvanic corrosion, the combination of two dissimilar metals with an electrolyte is all that is needed to form a. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web metals are rated in their ability to resist electrochemical corrosion on the scale of nobility and on the galvanic series chart, which shows their electrical potential in seawater. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Dissimilar metals used in conjunction; Web below is a galvanic reaction chart for dissimilar metals. Web galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical contact, in the. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web the common factors are dissimilar metals, electrical contact, and a conductive electrolyte in contact with them. Obviously galvanic corrosion is more. Web galvanic corrosion may be experienced when two dissimilar metals or alloys, not in direct contact, are nevertheless connected electrically. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes.
Metals Galvanic Compatibility Chart A Visual Reference of Charts
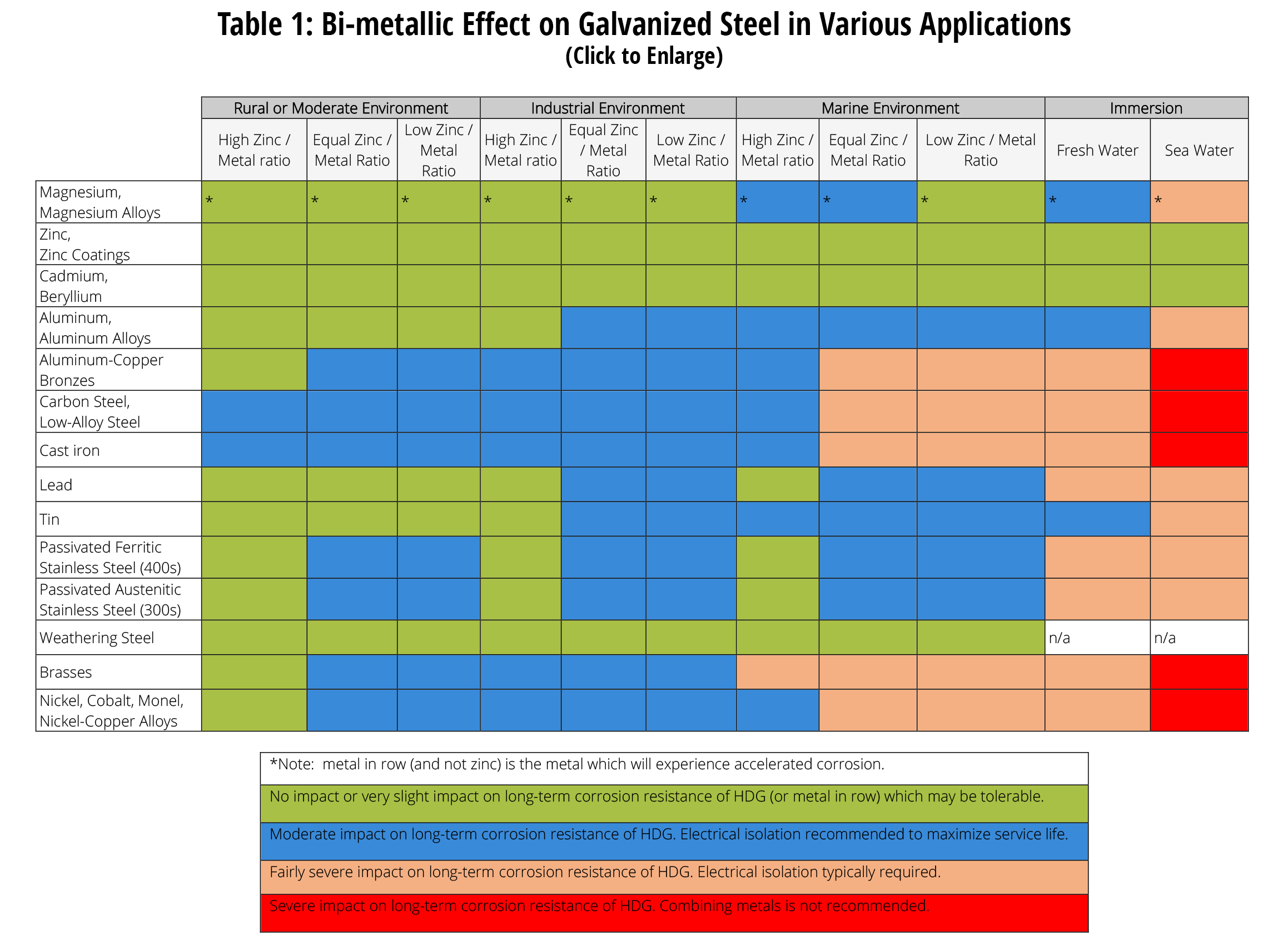
Dissimilar Metal Corrosion with… American Galvanizers Association
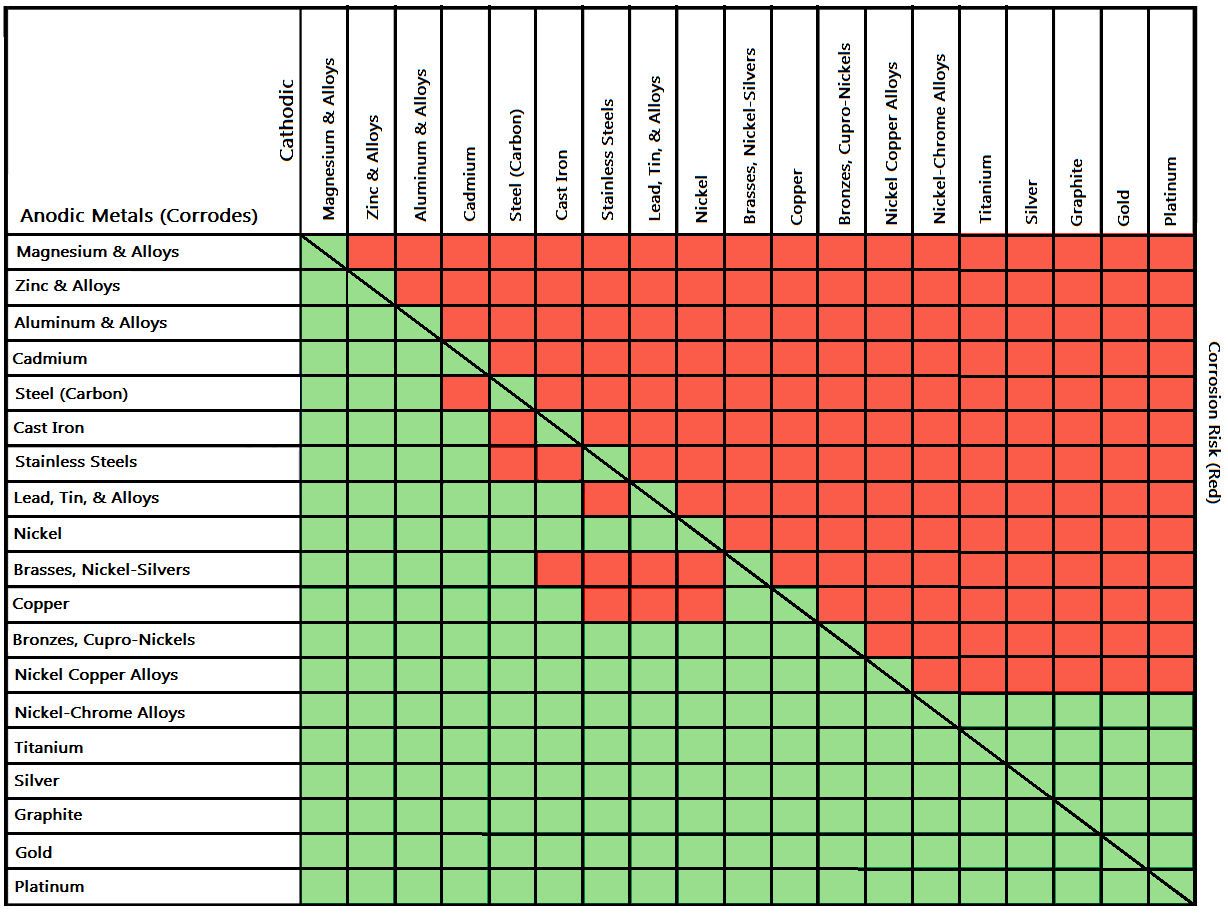
Galvanic Corrosion Common Questions Answered

FAQ 1 Galvanic/Dissimilar Metal Corrosion
Dissimilar Corrosion Materials Tables
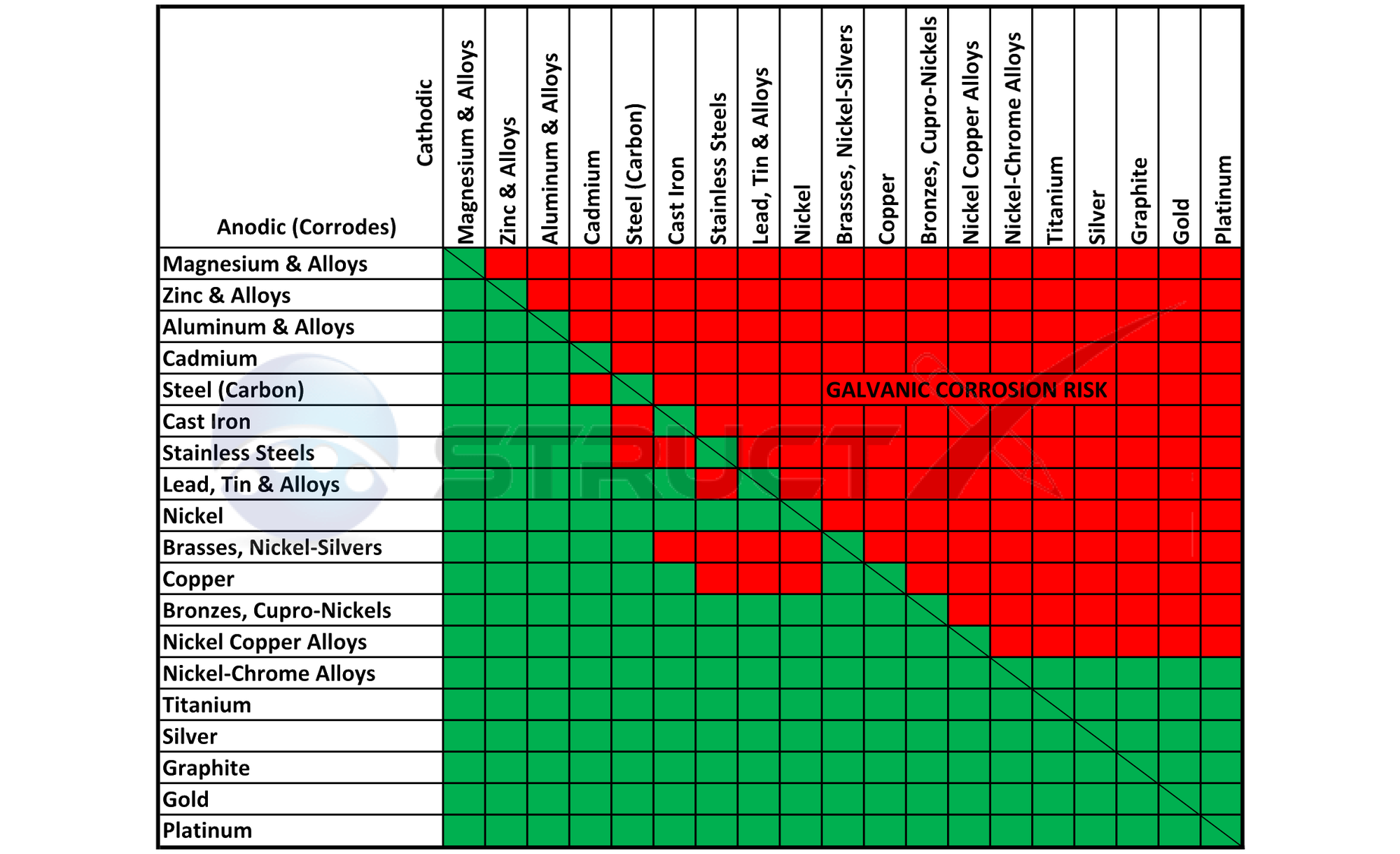
Aluminum Corrosion Resistance Chart

21 Lovely Galvanic Corrosion Chart Dissimilar Metals Chart Gallery

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot

Stainless Steel Galvanic Corrosion Chart

Protection of Dissimilar Metal Contacts and Corrosion Limits
Web However, You Can Completely Avoid Galvanic Corrosion By Choosing Matching Metal Anchors.
Contact A Corrosion Specialist To Determine The Best.
Galvanic Corrosion (Also Called Bimetallic Corrosion Or Dissimilar Metal Corrosion) Is An Electrochemical Process In Which One Metal Corrodes Preferentially When It Is In Electrical Contact With Another, In The Presence Of An Electrolyte.
Web Galvanic Corrosion Potential Is A Measure Of How Dissimilar Metals Will Corrode When Placed Against Each Other In An Assembly.
Related Post: