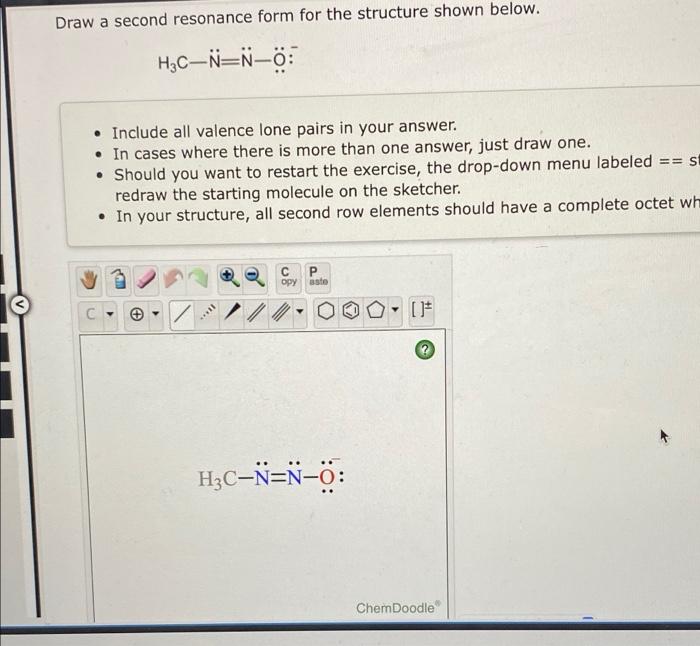
Draw A Second Resonance Form For The Structure Shown Below
Draw A Second Resonance Form For The Structure Shown Below - Add only the lone pairs found on all resonance structures. Draw a second resonance form for the structure shown below. Identify the atoms that can form double bonds. Hzc include all valence lone pairs in your answer. Web first, we need to identify the atoms that can move their electrons to form a double bond or a lone pair. Web the two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Add your answer and earn points. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. In this case, we have a nitrogen atom with a lone pair and a carbon atom with a double bond. Here’s the best way to solve it. Add only the lone pairs found on all resonance structures. Web draw the second resonance form for the structure shown below: This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. H3c include all valence lone pairs in your answer. To generate the structure shown below, start by drawing the base structure. Identify the atoms involved in resonance: In this case, we have a nitrogen atom with a lone pair and a carbon atom with a double bond. Rbowlin5700 is waiting for your help. • include all valence lone pairs in your answer. Identify the atoms that can form double bonds. • in cases where there is more than one answer, just draw one. Web the two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Web 100% (16 ratings) share share. Web draw a second resonance form. Draw a second resonance form for the structure shown below. • include all valence lone pairs in your answer. Web 100% (16 ratings) share share. H2c ch, draw a second resonance form for the structure shown below. Determine the relative stability of resonance structures using a set of rules. After completing this section, you should be able to. Identify the atoms involved in resonance: Web draw second resonance form for the structure shown below. Equivalent lewis structures are called resonance forms. This problem has been solved! Draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown below. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Draw a second resonance form for the structure shown below. You'll get a detailed solution from a subject matter expert that helps you. Draw a second resonance form for the structure shown below. Web the two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Later, we will show that the contributor with the negative charge on. Web draw a second resonance form for the structure shown below.. Next, add the first peak in the center of the base structure. Ch2 сн3 draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown below. Use the concept of resonance to explain structural features of molecules and ions. Web a resonance form is another way of drawing a lewis dot structure. Web draw a second resonance form for the structure shown below. Here’s the best way to solve it. • in cases where there is more than one answer, just draw one. Web the two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Web draw. H2c ch2 draw a second resonance form for the structure shown below. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. After completing this section, you should be able to. Here’s the best way to solve it. Draw the resonance structures of molecules or ions that exhibit delocalization. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw a second resonance form for the structure shown below. Web draw the second resonance form for the structure shown below: Jonathan and his sister jennifer have a combined. Add only the lone pairs found on all resonance structures. Later, we will show that the contributor with the negative charge on. • in cases where there is more than one answer, just draw one. Hzc include all valence lone pairs in your answer. • include all valence one pairs in your answer. Web draw a second resonance form for the structure shown below. In this case, we have a carbon atom with a double bond to an oxygen atom, and a hydrogen atom bonded to the carbon atom.step. Here’s how to approach this question. Web 100% (16 ratings) share share. Identify the location of delocalizable π electrons or lone pairs on the atoms in the. Draw a second resonance form for the structure shown below.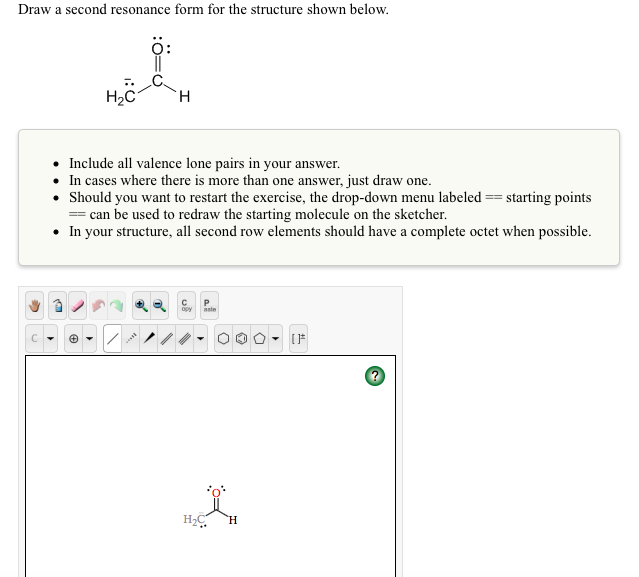
Solved Draw a second resonance form for the structure shown

Draw a Second Resonance Form for the Structure Shown Below. TrendingWorld

Solved Draw a second resonance form for the structure shown
[Solved] Draw a second resonance form for the structure shown below. H
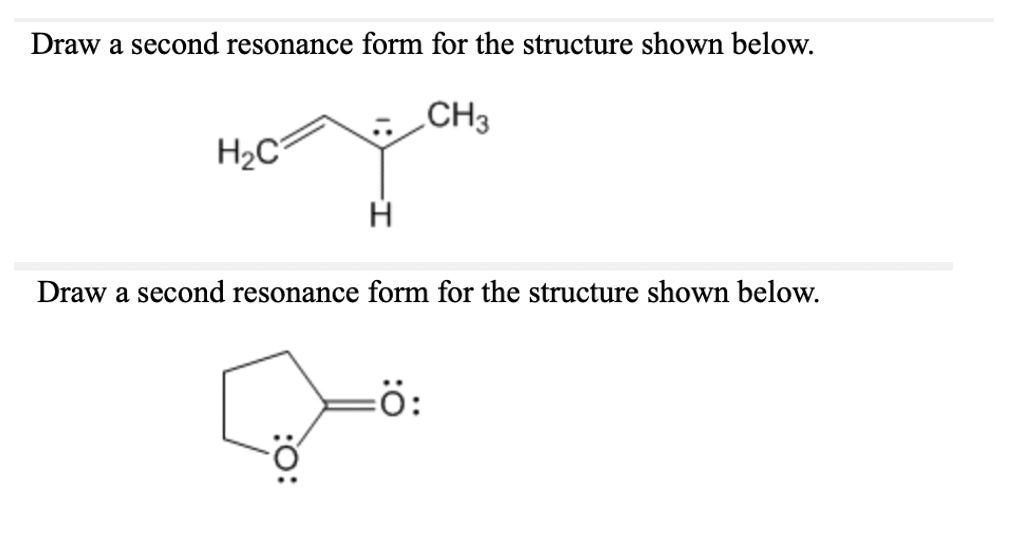
SOLVED Draw a second resonance form for the structure shown below CH3
Solved Draw a second resonance form for the structure shown

OneClass Draw a second resonance form for the structure shown below
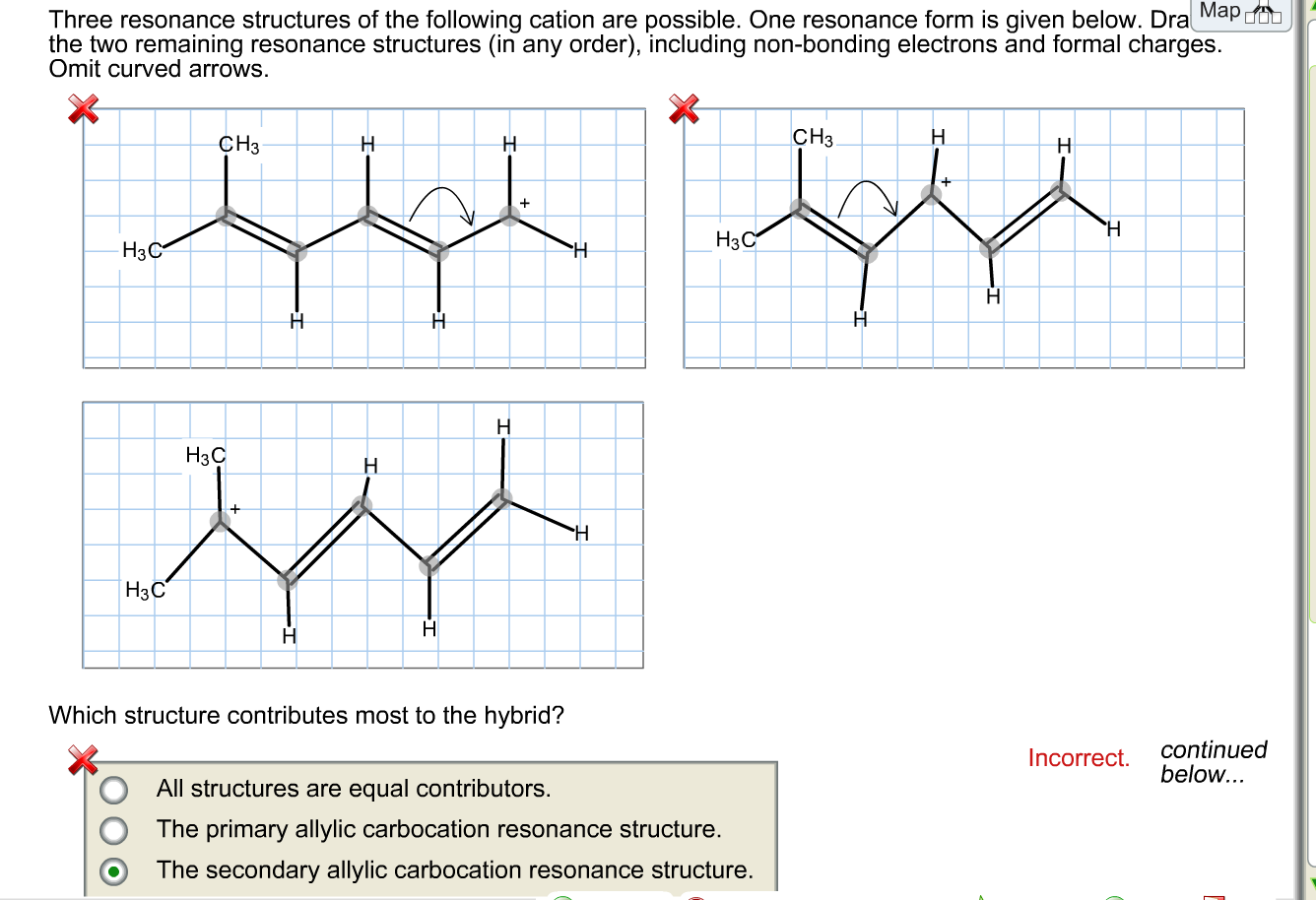
draw two resonance structures of the cation shown below
[Solved] Draw a second resonance form for the structure shown below. O
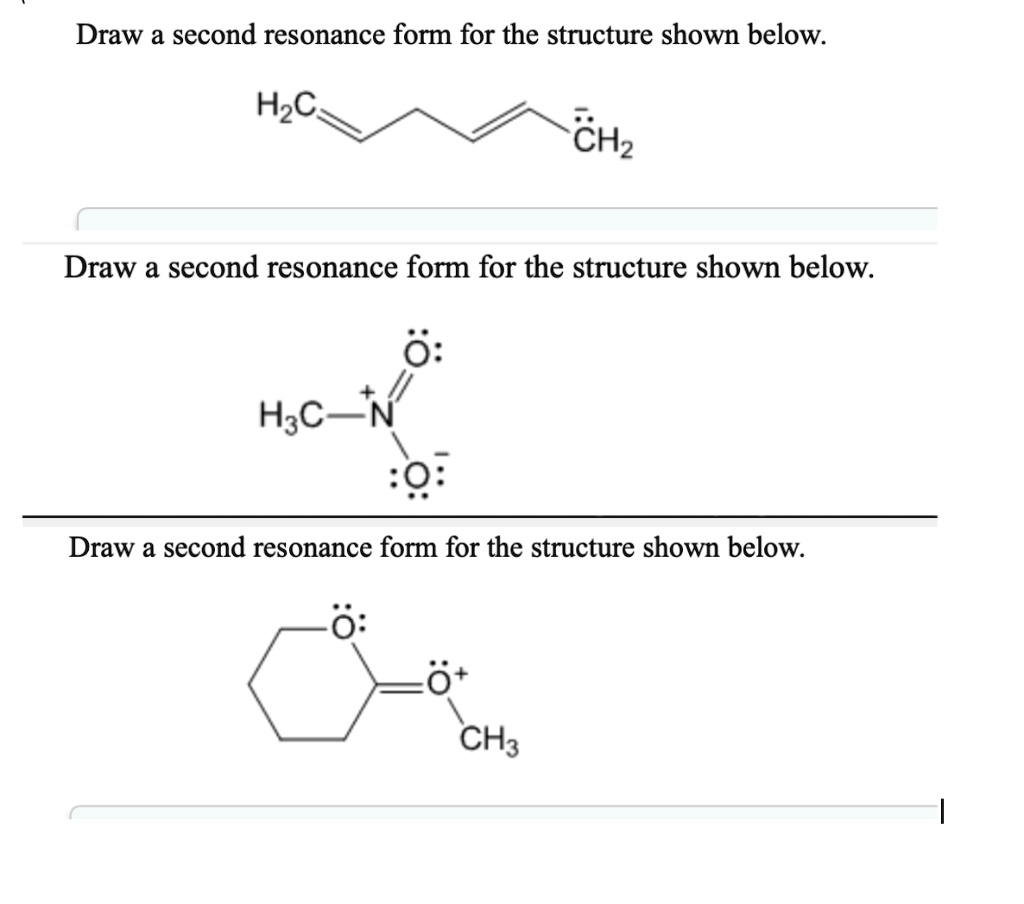
SOLVED Draw a second resonance form for the structure shown below H3C
Web Draw A Second Resonance Form For The Structure Shown Below.
Next, Add The First Peak In The Center Of The Base Structure.
In This Case, We Have A Nitrogen Atom With A Lone Pair And A Carbon Atom With A Double Bond.
• In Cases Where There Is More Than One Answer, Just Draw One.
Related Post: