Draw Sodium Atom
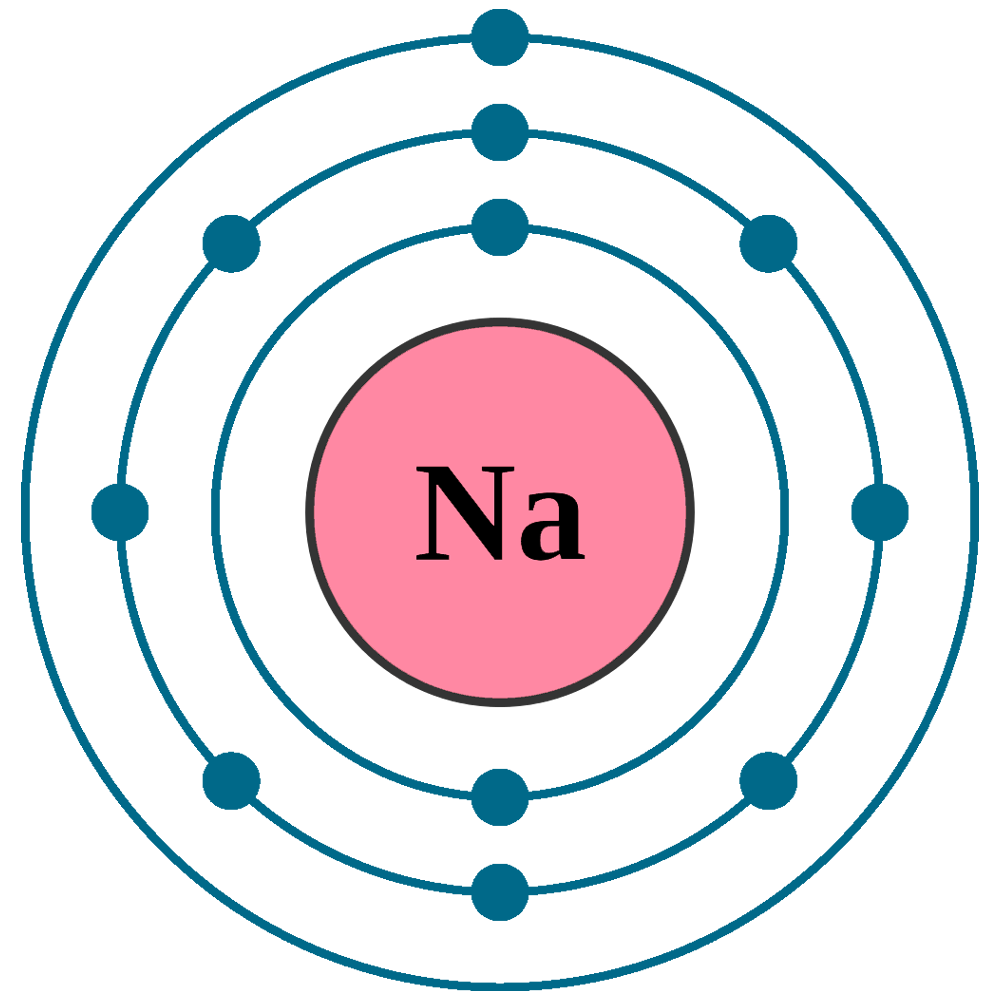
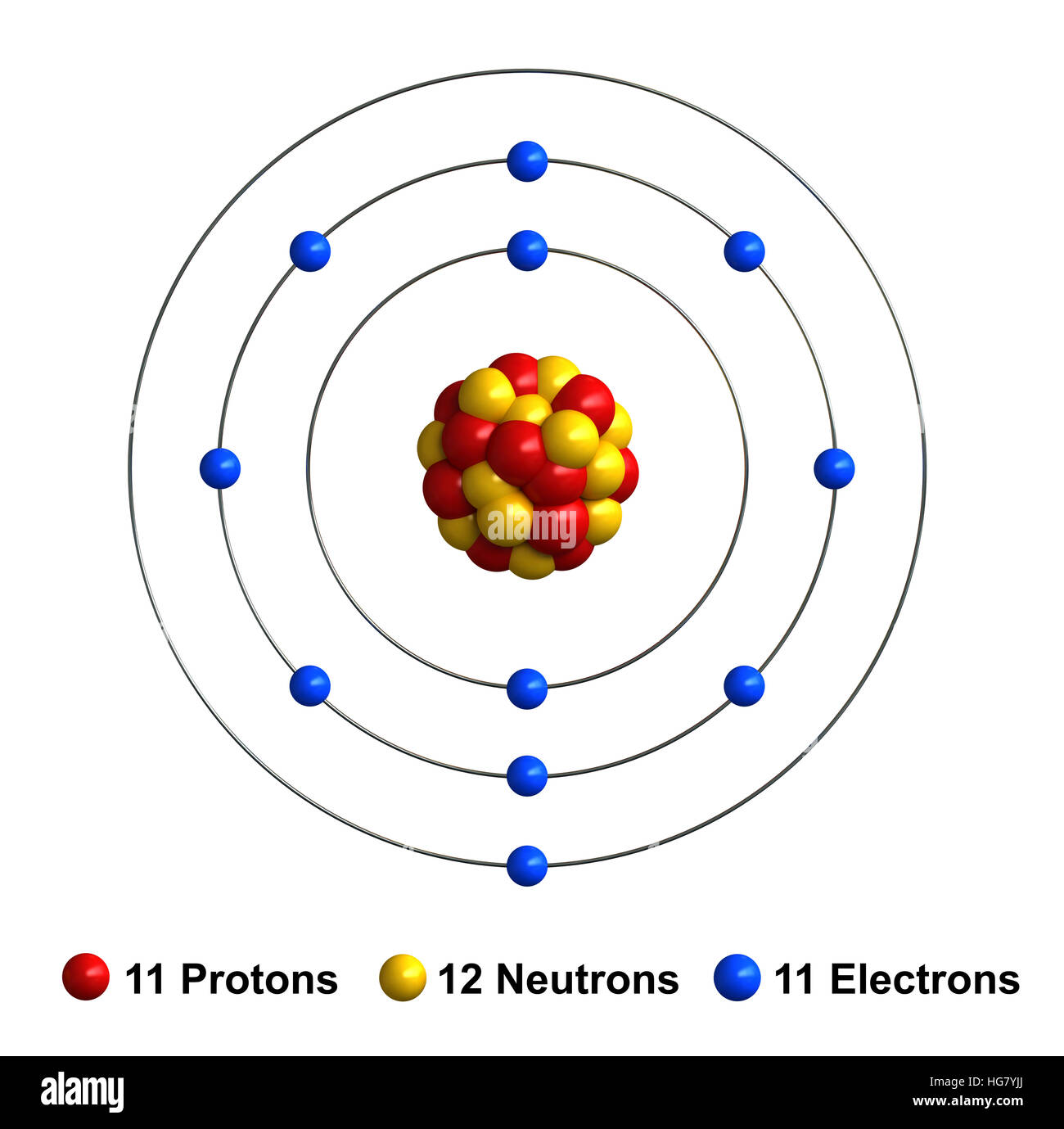
Draw Sodium Atom - The electronic configuration of sodium is: Electron shells niels bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. Sodium is neutral and its atomic number is 11, hence, the number of protons and electrons available for its bohr diagram is also 11. Learn how bohr models are used to represent atoms. Models help us visualize atomic structure. This video shows how to draw the orbital diagram of sodium (na). The valence shell of the sodium atom is. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Web complete step by step answer: Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 1 electron. This video shows how to draw the orbital diagram of sodium (na). 32k views 9 years ago. Web therefore the sodium electron configuration will be 1s 2 2s. Sodium is an atom in the periodic table with atomic number. Web the electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). It also shows how to write the electron configuration of sodium (na) and the shorthand noble gas. The number of neutrons = Web the bohr model of sodium (na) is drawn with three electron shells, the. Web 140k views 5 years ago. Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 1 electron. A picture of a hydrogen atom can be found here. High school chemistry > the bohr model. Web draw the bohr diagram. The valence shell of the sodium atom is. It has a total of. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. 11), the most common isotope of the element sodium. Learn. Sodium is an atom in the periodic table with atomic number. 11), the most common isotope of the element sodium. Electron shells niels bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. It is in group 1 of the periodic table. 1 s 2 2 s 2. 11), the most common isotope of the element sodium. The number of neutrons = Atoms are way too small to see with the naked eye (and even most microscopes). Sodium is neutral and its atomic number is 11, hence, the number of protons and electrons available for its bohr diagram is also 11. 32k views 9 years ago. Web therefore the sodium electron configuration will be 1s 2 2s 2 2p 6 3s 1. 11 electrons (green) bind to the nucleus, with a single, relatively unstable electron in the outer shell (ring). 32k views 9 years ago. The number of dots equals the number of valence electrons in the atom. What happens when a sodium atom becomes a. The valence shell of the sodium atom is. 32k views 9 years ago. Web 140k views 5 years ago. High school chemistry > the bohr model. Web draw the bohr diagram of an atom with 18 electrons or fewer. Web the electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). The configuration notation provides an easy way for scientists to write and communicate how electrons. 32k views 9 years ago. The number of neutrons = High school chemistry > the bohr model. The nucleus consists of 11 protons (red) and 12 neutrons (blue). Web the electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). Atoms are way too small to see with the naked eye (and even most microscopes). 1 s 2 2 s 2 2 p 6 3 s 1. The number of dots equals the number of valence. Atoms are way too small to see with the naked eye (and even most microscopes). The electronic configuration of sodium is: 11), the most common isotope of the element sodium. It is in group 1 of the periodic table. Electron shells niels bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. The configuration notation provides an easy way for scientists to write and communicate how electrons. 1 s 2 2 s 2 2 p 6 3 s 1. Web therefore the sodium electron configuration will be 1s 2 2s 2 2p 6 3s 1. So, we represent atoms using models. Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 1 electron. The nucleus consists of 11 protons (red) and 12 neutrons (blue). Models help us visualize atomic structure. Web complete step by step answer: It also shows how to write the electron configuration of sodium (na) and the shorthand noble gas. The valence shell of the sodium atom is. This video shows how to draw the orbital diagram of sodium (na).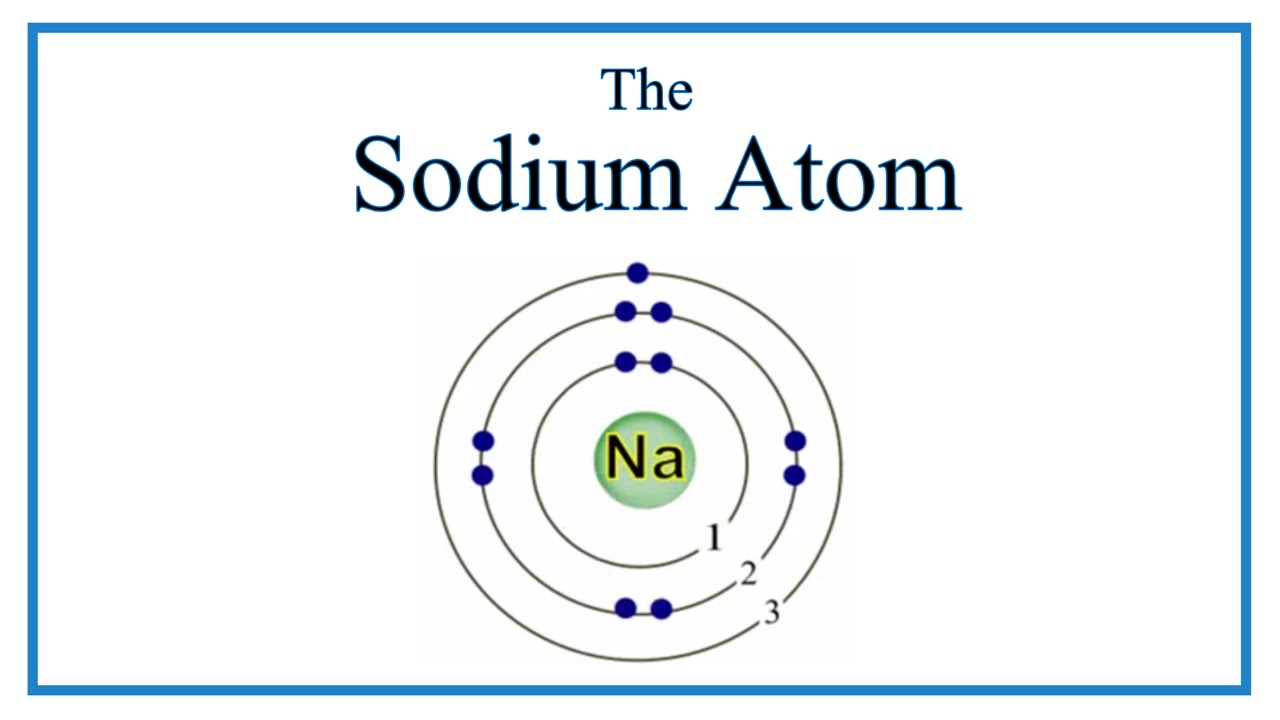
Atomic Structure of the Sodium Atom (Na) YouTube
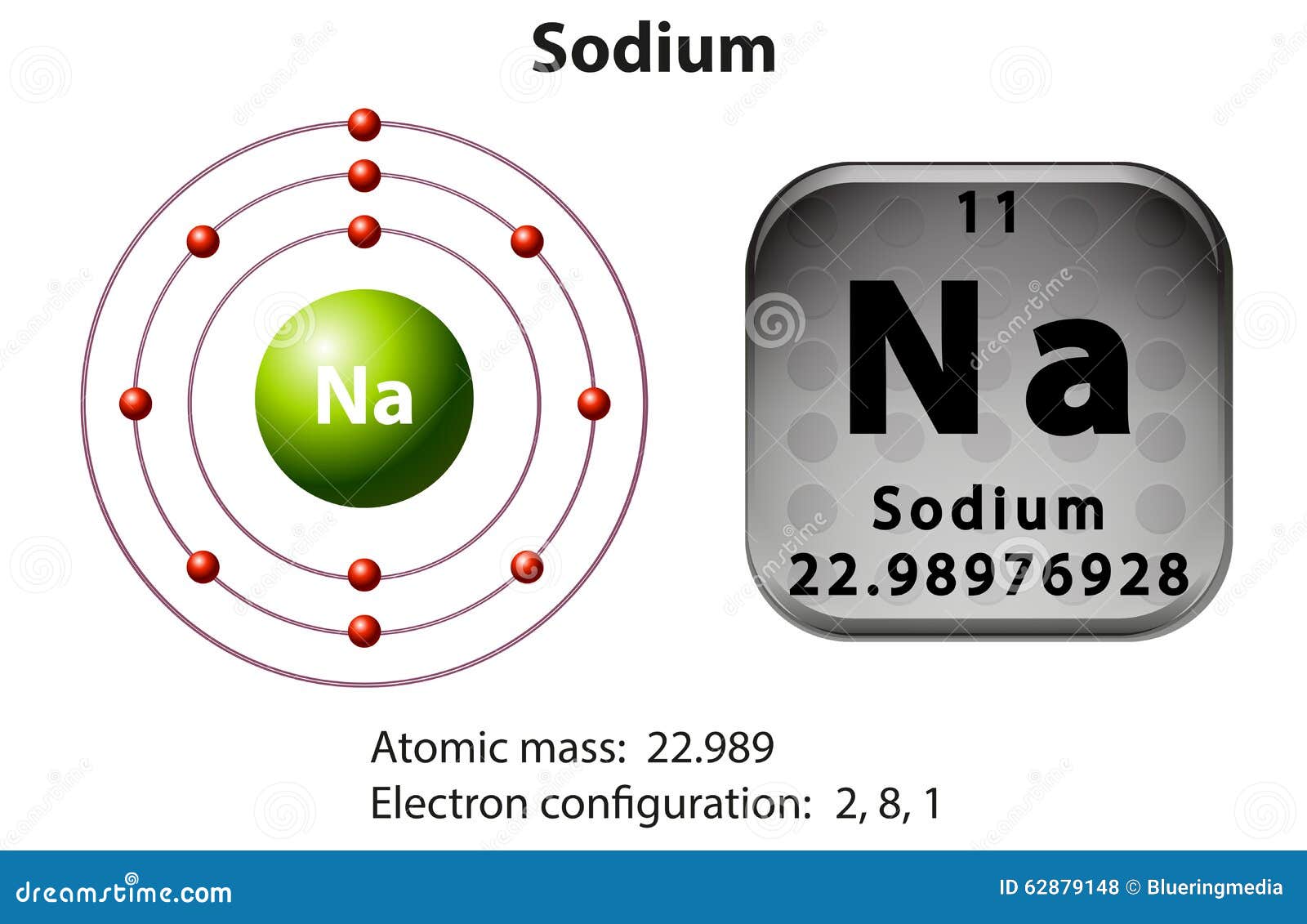
Symbol and Electron Diagram for Sodium Stock Vector Illustration of
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
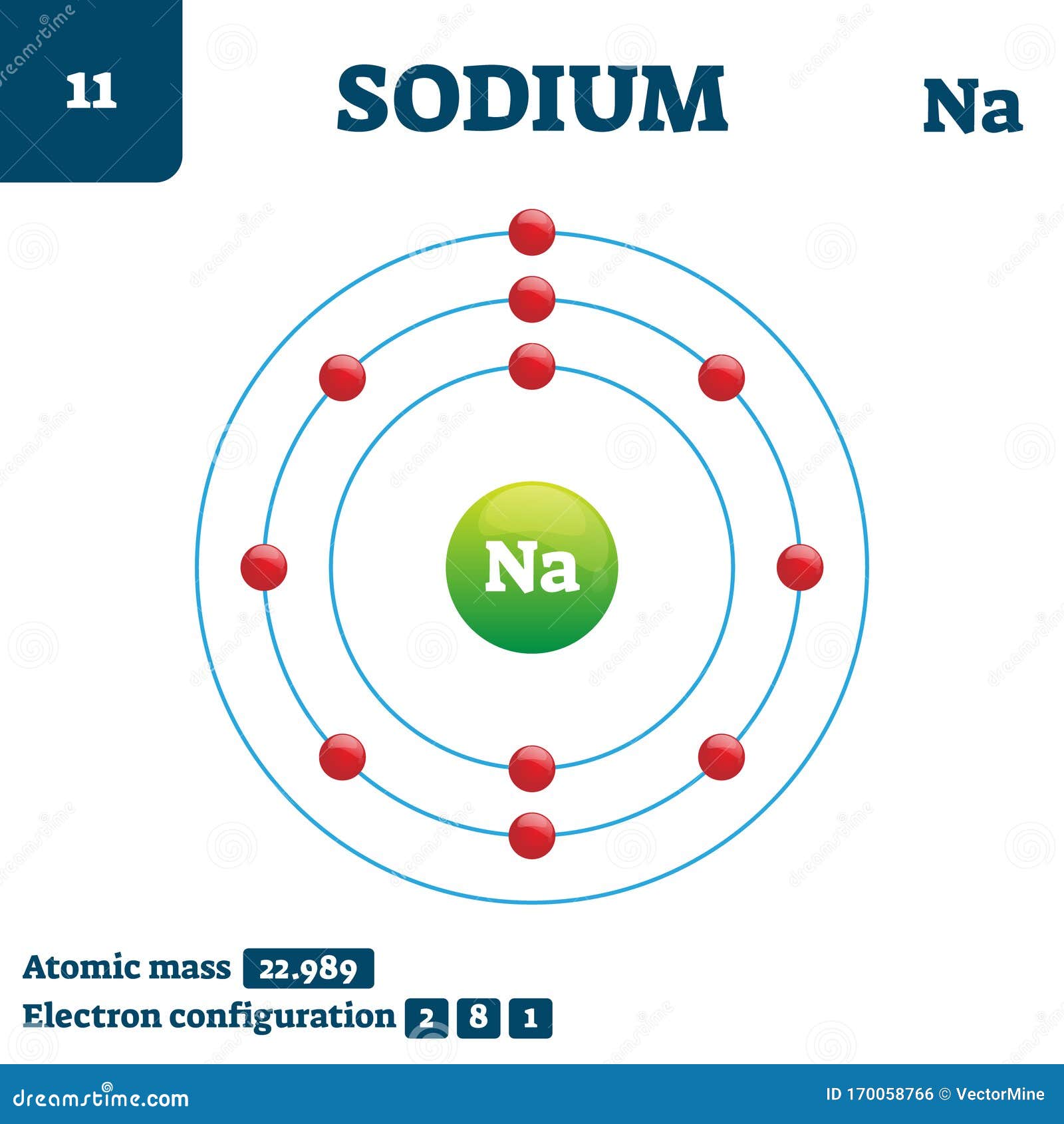
Sodium Chemical Element, Vector Illustration Diagram Stock Vector

FileElectron shell 011 sodium.png Wikimedia Commons
Sodium Na (Element 11) of Periodic Table NewtonDesk
:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)
Atom Diagrams Electron Configurations of the Elements
Sodium Atomic Structure High Resolution Stock Photography and Images
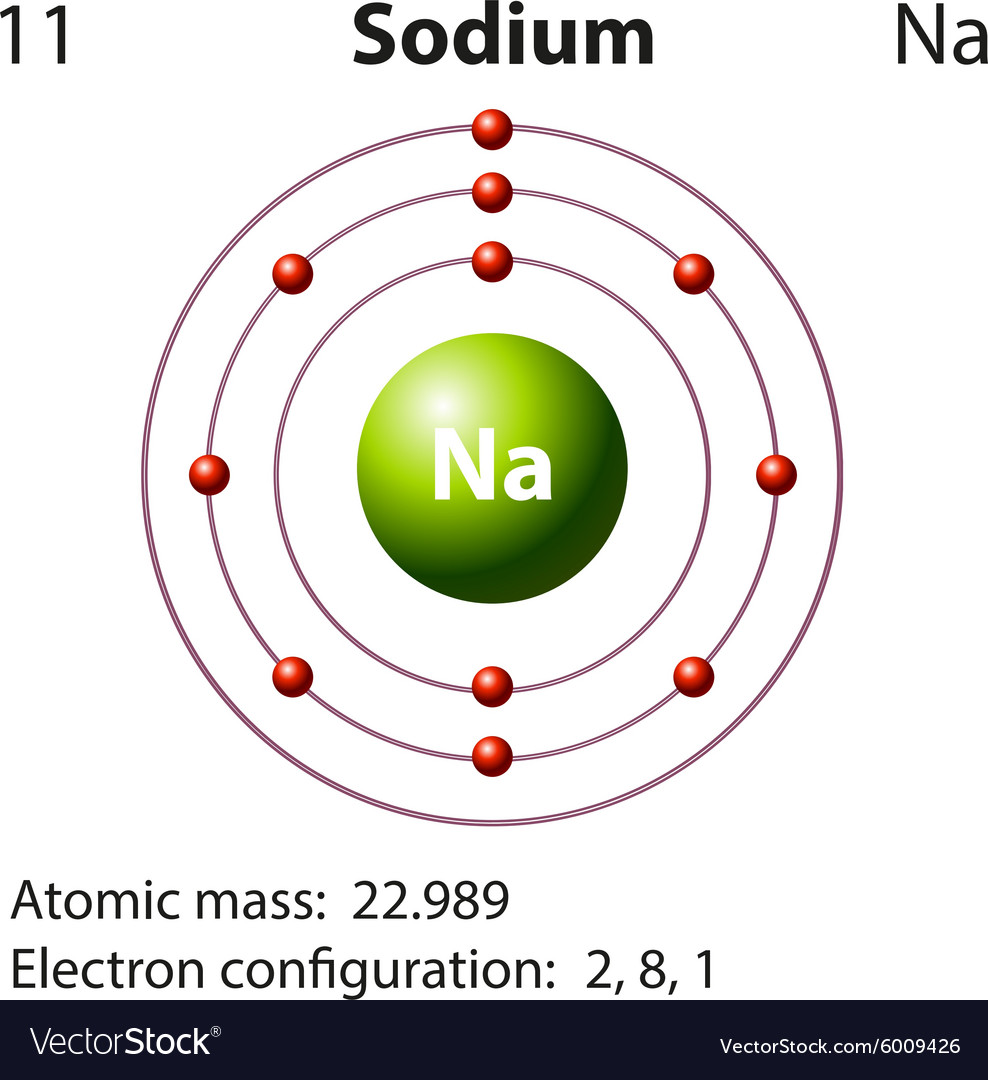
Diagram representation of the element sodium Vector Image

The sodium atom YouTube
11 Electrons (Green) Bind To The Nucleus, With A Single, Relatively Unstable Electron In The Outer Shell (Ring).
High School Chemistry > The Bohr Model.
Web The Electron Configuration Of Sodium Is \(1S^2 2S^2 2P^6 3S^1\) (Table \(\Pageindex{1}\)).
For The Na+ Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For.
Related Post: