Draw The Electron Configuration For A Neutral Atom Of Iron
Draw The Electron Configuration For A Neutral Atom Of Iron - Therefore, its ground state electronic configuration can be. Web we add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Electron configurations describe where electrons are located around the nucleus of an atom. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Web what is the electron configuration of: Draw the electron configuration for a neutral atom of iron. Draw the electron configuration for a neutral atom of iron. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 the electron configuration of a neutral atom of iron is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6. When looking at electron configuration, your fill order of electrons is: Draw the electron configuration for a neutral atom of iron. Web chemistry questions and answers. Using the aufbau principle, the pauli exclusion principle, and hund's. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Draw the electron configuration for a neutral atom of iron. Use the shortcut method for writing electron configuration codes for atoms and ions. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the. The number of dots equals. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Group 1a (1), the alkali metals all end is s1. Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number. Web to write electron configurations and draw orbital box diagrams, there are three rules that must be applied. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web we add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web in order to. Group 1a (1), the alkali metals all end is s1. Once we have the configuration for fe, the ions are simple. Draw the electron configuration for a neutral atom of iron. Therefore, its ground state electronic configuration can be. This problem has been solved! Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Note that the total number of electrons in the neutral atom adds up to the atomic number, so 2+2+6+2+6 = 18, which is the atomic number of ar. Web here, the electron configuration of. The element atomic number and name are listed in the upper left. Web to determine the electron configuration of iron, we need to fill up the energy levels in order of increasing energy. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web what is the electron configuration of: Electrons are represented by. Differentiate between (spdf) electron configuration, orbital. Draw the electron configuration for a neutral atom of iron. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The number of dots equals. Want to join the conversation? Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web here, the electron configuration of iron ion(fe 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. This is the electron configuration of helium; This is sometimes called the bohr, or the ‘solar system’,. Draw the electron configuration for a neutral atom of iron. The aufbau principle states that electrons are always placed in the lowest energy sublevel that is available. Use the shortcut method for writing electron configuration codes for atoms and ions. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features. Draw the electron configuration for a neutral atom of iron. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. It denotes a full s orbital. Group 1a (1), the alkali metals all end is s1. The iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ion(fe 3+ ). Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Shortcut for writing electron configurations. Web to determine the electron configuration of iron, we need to fill up the energy levels in order of increasing energy. Web here, the electron configuration of iron ion(fe 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. Web first we determine the number of electrons in the atom; Both are explained in the video below. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The upper right side shows the number of electrons in a neutral atom. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The number of dots equals. Draw the electron configuration for a neutral atom of iron.
Orbital Diagram Of Iron

Iron Protons Neutrons Electrons Electron Configuration

Draw the electron configuration for a neutral atom of iron. Quizlet
Solved Draw the electron configuration for a neutral atom of

How to Find the Valence Electrons for Iron (Fe)?

Electronic Configuration How To Write Electron ConfigurationChemistry
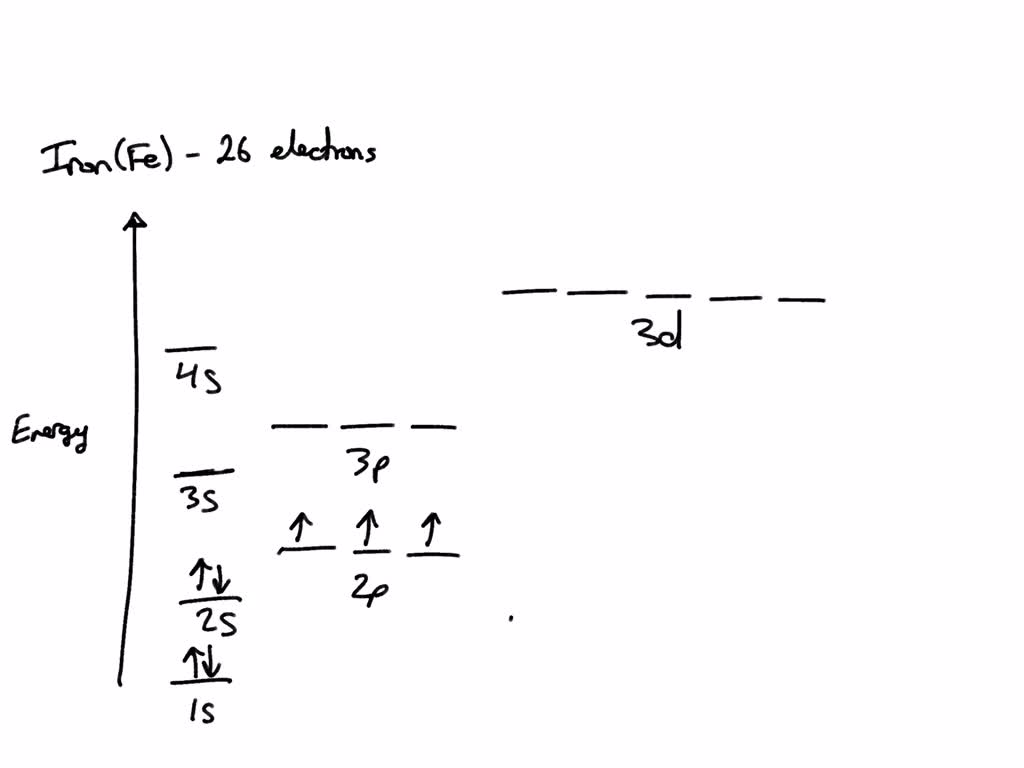
SOLVED Draw the electron configuration for a neutral atom of iron energy
Solved Draw the electron configuration for a neutral atom of

Iron electronic configuration How to Write Iron electronic
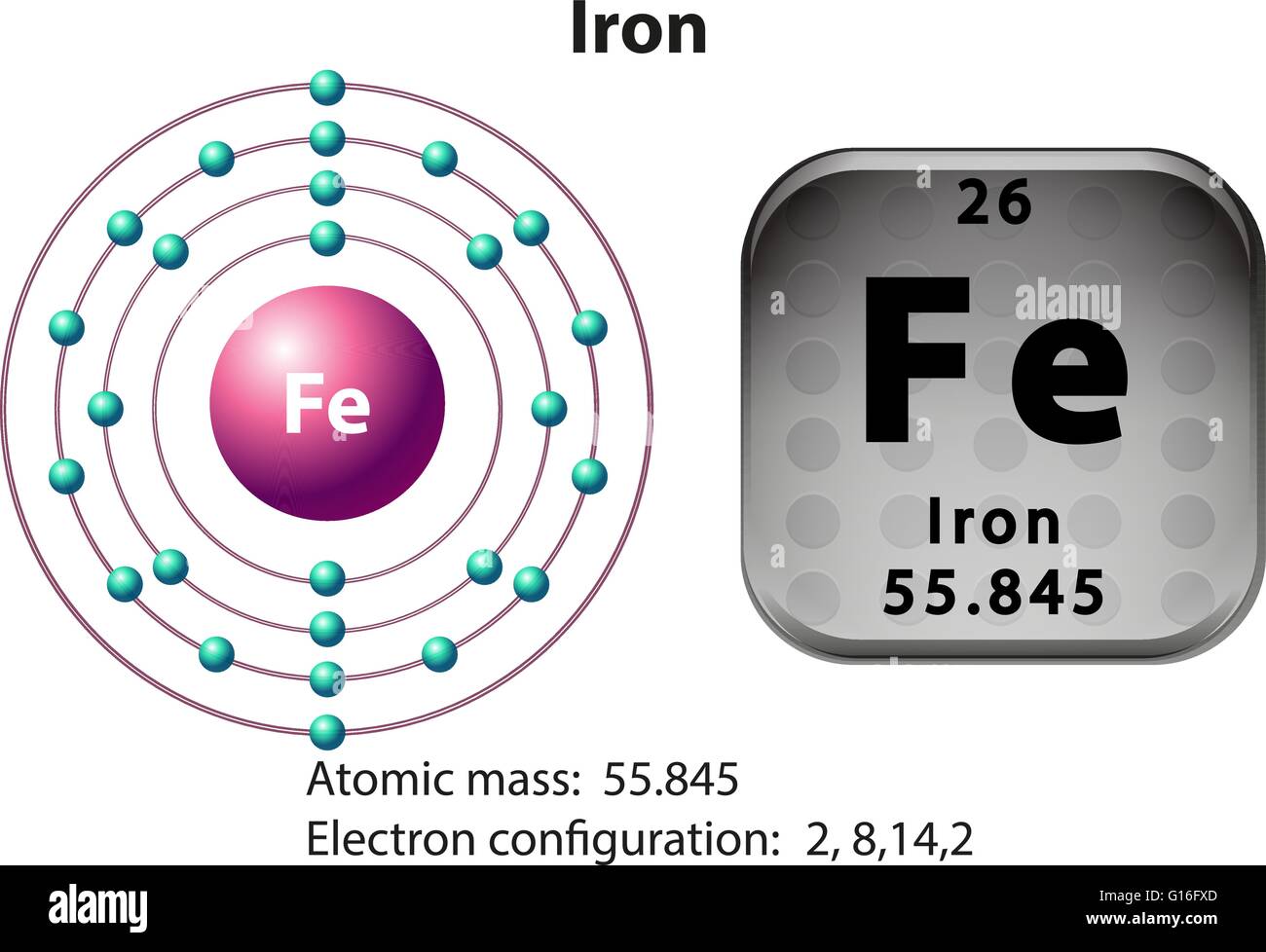
Symbol and electron diagram for Iron illustration Stock Vector Image
This Is Sometimes Called The Bohr, Or The ‘Solar System’, Model.
The Element Atomic Number And Name Are Listed In The Upper Left.
Distinguish Between Outer Energy Level (Valence) Electrons And Core Electrons.
Use The Shortcut Method For Writing Electron Configuration Codes For Atoms And Ions.
Related Post: