Draw The Lewis Dot Diagram For A Cation
Draw The Lewis Dot Diagram For A Cation - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web lewis symbols can be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: 37k views 5 years ago. 54k views 5 years ago. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. There are 2 steps to solve this one. Identify the atomic number of arsenic (as) which is 33, and understand its electronic. Shared pairs of electrons are drawn as lines between atoms, while lone pairs. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Draw a lewis electron dot diagram for an atom or a monatomic ion. And putting a positive charge outside of it. Draw the lewis dot diagram for a k™ cation. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. 28k views 3 years ago. Note that hydrogen is often shown in both. Remember that lewis dot structures. 140k views 5 years ago. 54k views 5 years ago. Web draw the lewis dot diagram for a h+ cation. Web draw lewis structures for ionic compounds. So there's your xenon pentafluoride cation. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block). Draw lewis structures for ionic compounds. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block). Note that hydrogen is often shown in both. Web draw the lewis dot diagram for a cl2+ cation. Draw lewis structures for ionic compounds. Once we know how many valence electrons there. 100% (4 ratings) share share. The diagram is also called a lewis. For the na+ structure use the periodic table to find the total number of. Draw a lewis electron dot diagram for an atom or a monatomic ion. For the na+ structure use the periodic table to find the total number of. 28k views 3 years ago. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. 54k views 5 years ago. 37k views 5 years ago. Web draw lewis structures for ionic compounds. 28k views 3 years ago. Shared pairs of electrons are drawn as lines between atoms, while lone pairs. For the k+ structure use the periodic table to find the total number of valence electrons for k. There are 2 steps to solve this one. For the na+ structure use the periodic table to find the total number of. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. 28k views 3 years ago. Web lewis electron dot diagrams use dots to represent valence electrons. Draw the lewis dot diagram for a k™ cation. 140k views 5 years ago. Lewis electron dot diagrams for ions have less (for cations) or more (for. And putting a positive charge outside of it. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block). 54k views 5 years ago. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Lewis electron dot diagrams for ions have less (for cations) or more (for. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and. So there's your xenon pentafluoride cation. 140k views 5 years ago. Web draw lewis structures for ionic compounds. Once we know how many valence electrons there. There are 2 steps to solve this one. Draw a lewis electron dot diagram for an atom or a monatomic ion. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block). Shared pairs of electrons are drawn as lines between atoms, while lone pairs. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In almost all cases, chemical bonds are formed by interactions of valence electrons in. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. This problem has been solved! Likewise, they can be used to show the formation of anions from. Draw lewis structures for ionic compounds. Web draw the lewis dot diagram for a as+ cation. Draw the lewis dot diagram for a k™ cation.
How to Draw a Lewis Structure
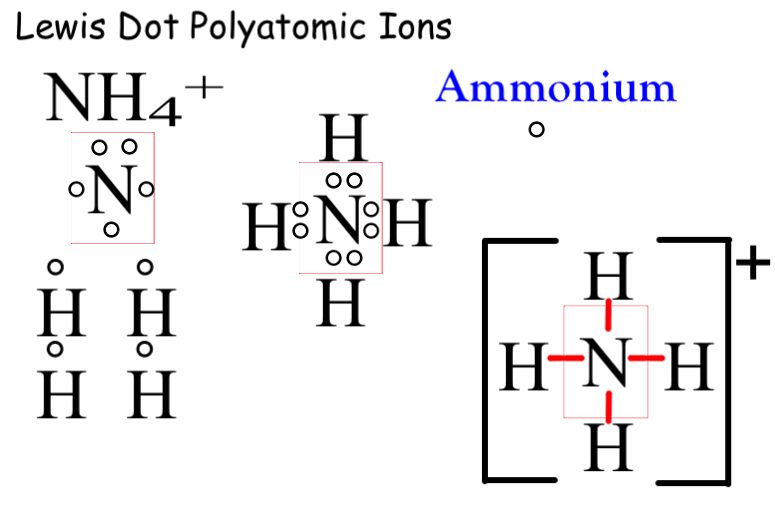
How do you draw lewis structures for polyatomic ions? Socratic

Lewis Dot Structures of Atoms and Ions YouTube

Lewis Structure Types

How To Draw Lewis Dot Diagrams Simplereality27
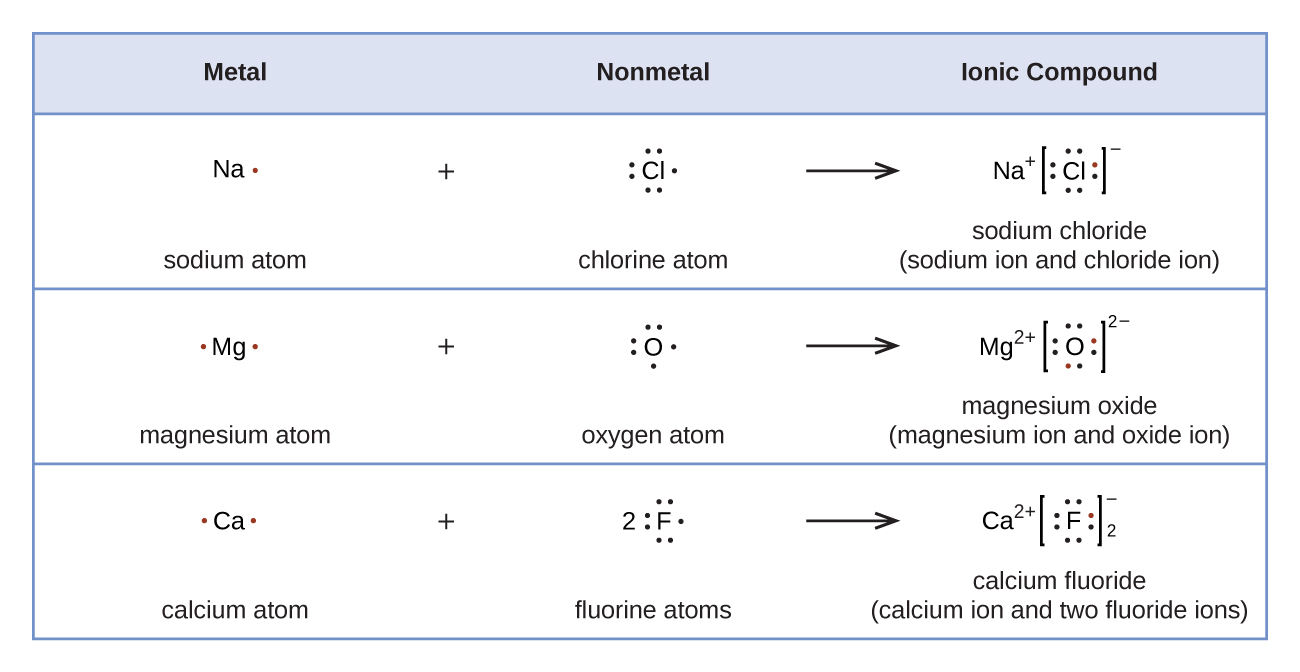
4.1 Lewis Dot Diagrams Chemistry LibreTexts

3 Ways to Draw Lewis Dot Structures wikiHow

Drawing Lewis Structures Chemistry Socratic
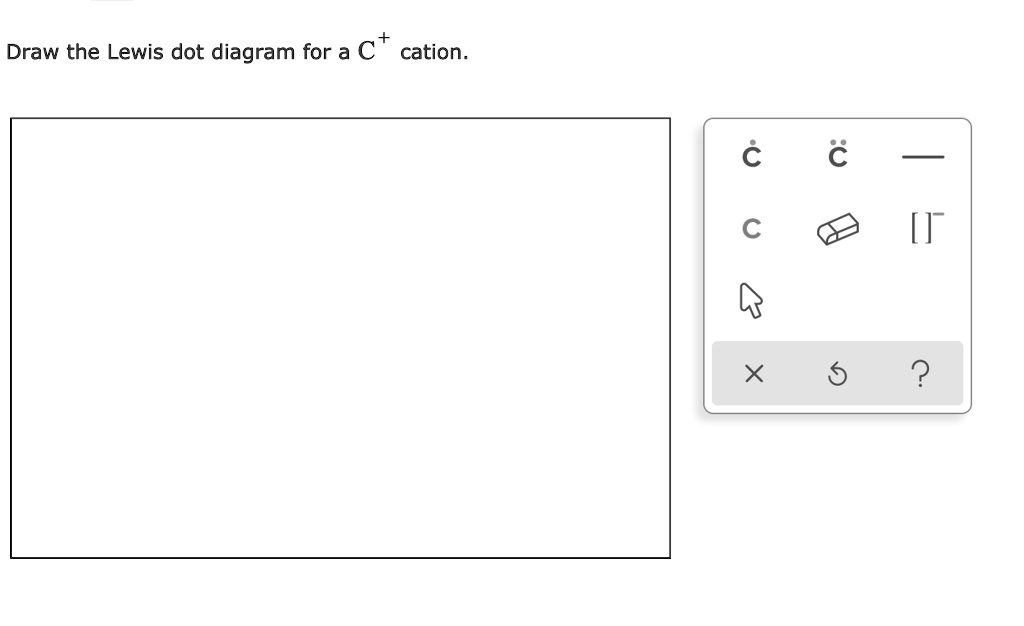
SOLVED Draw the Lewis dot diagram for a C cation. 2
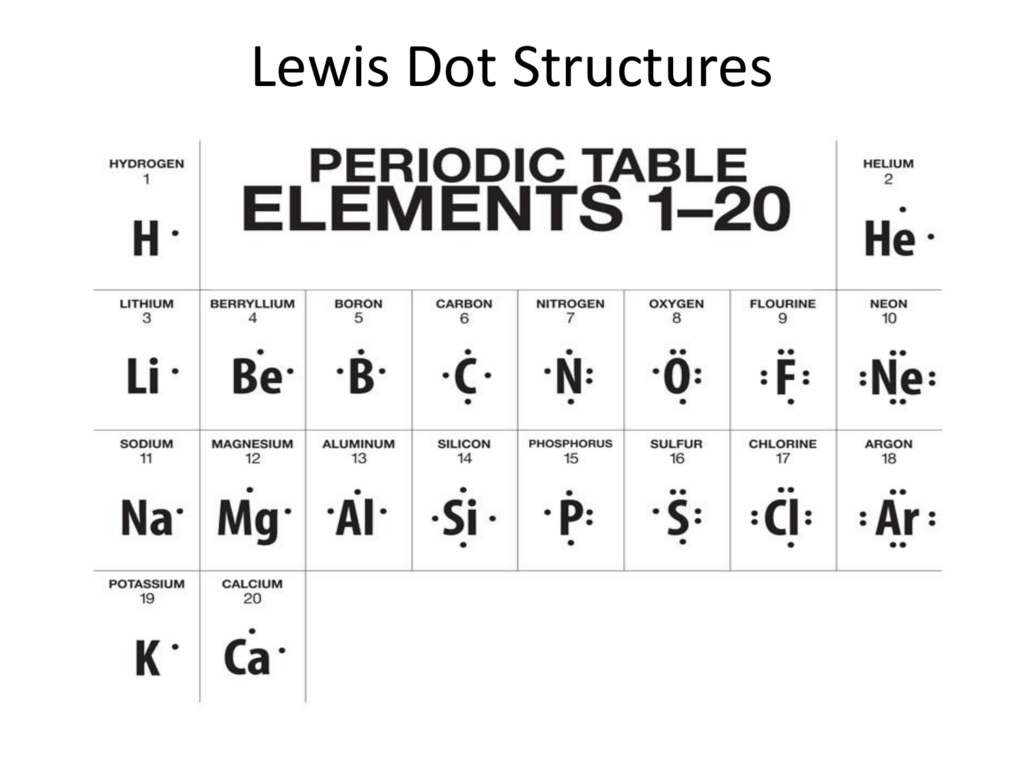
Lewis Dot Structures
Web Draw The Lewis Dot Diagram For A H+ Cation.
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Remember That Lewis Dot Structures.
100% (4 Ratings) Share Share.
Related Post: