Draw The Lewis Dot Structure For No
Draw The Lewis Dot Structure For No - 216k views 6 years ago. For the no+ structure use the periodic. Web the formal charge on each atom is: I understand the valence electron part of it, but i don't understand the actual drawing of the lewis structure. You'll want to calculate the formal. A lewis structure is a diagram that shows the chemical bonds between atoms in a. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web nitric oxide is a simple molecule which contains only two atoms; The lewis structure for no requires you to place fewer than 8 valence electrons on nitrogen (n). Drawing the lewis structure for no +. The lewis structure for no requires you to place fewer than 8 valence electrons on nitrogen (n). Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. 323k views 4 years ago 1 product. You'll want to calculate the formal. Web draw out a correct lewis structure for the following compounds. Follow these simple steps to draw lewis dot structures: The example is for the nitrate ion. Web this widget gets the lewis structure of chemical compounds. I'll cover how to properly draw lewis structures of regular molecules and lewis structures of ions. You'll want to calculate the formal. For the no+ structure use the periodic. The example is for the nitrate ion. Draw the atoms on paper and put dots. Due to its simple structure (no has only two atoms), we can easily draw the lewis structure. The lewis structure for no requires you to place fewer than 8 valence electrons on nitrogen (n). Web this widget gets the lewis structure of chemical compounds. Web nitric oxide is a simple molecule which contains only two atoms; Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web drawing the lewis structure for no. I understand the valence electron part of it, but i don't understand the actual drawing of. Draw the lewis structure for no. I'll cover how to properly draw lewis structures of regular molecules and lewis structures of ions. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web draw out a correct lewis structure for the following compounds. Drawing the lewis structure for no +. A lewis structure is a diagram that shows the chemical bonds between atoms in a. Web drawing the lewis structure for no. Web draw out a correct lewis structure for the following compounds. Web drawing lewis dot structures and resonance structures. Web for example, the lewis electron dot diagram for hydrogen is simply \[\mathbf{h}\mathbf{\cdot}\nonumber \] because the side is not. Web drawing the lewis structure for no. The example is for the nitrate ion. For the no+ structure use the periodic. Web draw out a correct lewis structure for the following compounds. Web nitric oxide is a simple molecule which contains only two atoms; Web for example, the lewis electron dot diagram for hydrogen is simply \[\mathbf{h}\mathbf{\cdot}\nonumber \] because the side is not important, the lewis. Drawing the lewis structure for no +. Web so what would be the lewis dot structure for the compound of n2o2? Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web drawing. A lewis structure is a diagram that shows the chemical bonds between atoms in a. Web nitric oxide is a simple molecule which contains only two atoms; A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Draw the molecule by placing atoms on the grid and connecting them with bonds. I'll cover how. Follow these simple steps to draw lewis dot structures: The lewis structure for no requires you to place fewer than 8 valence electrons on nitrogen (n). Web nitric oxide is a simple molecule which contains only two atoms; Draw the lewis structure for no. The example is for the nitrate ion. Web drawing the lewis structure for no. Web so what would be the lewis dot structure for the compound of n2o2? Web draw out a correct lewis structure for the following compounds. Drawing the lewis structure for no +. Web here are the steps to draw a lewis structure. Due to its simple structure (no has only two atoms), we can easily draw the lewis structure. Draw the lewis structure for no. I understand the valence electron part of it, but i don't understand the actual drawing of the lewis structure. Web this widget gets the lewis structure of chemical compounds. 216k views 6 years ago. Web for example, the lewis electron dot diagram for hydrogen is simply \[\mathbf{h}\mathbf{\cdot}\nonumber \] because the side is not important, the lewis. A lewis structure is a diagram that shows the chemical bonds between atoms in a. With no + be sure to remove a valence. Draw the atoms on paper and put dots. You'll want to calculate the formal. I'll cover how to properly draw lewis structures of regular molecules and lewis structures of ions.
3 Ways to Draw Lewis Dot Structures wikiHow

3 manières de dessiner une représentation de Lewis

Molecular Modeling Digital and Analog Middlebury College Chem 103 lab

Lewis Dot Structure Definition, Examples, and Drawing

Draw the Lewis Structure of NO(+) YouTube
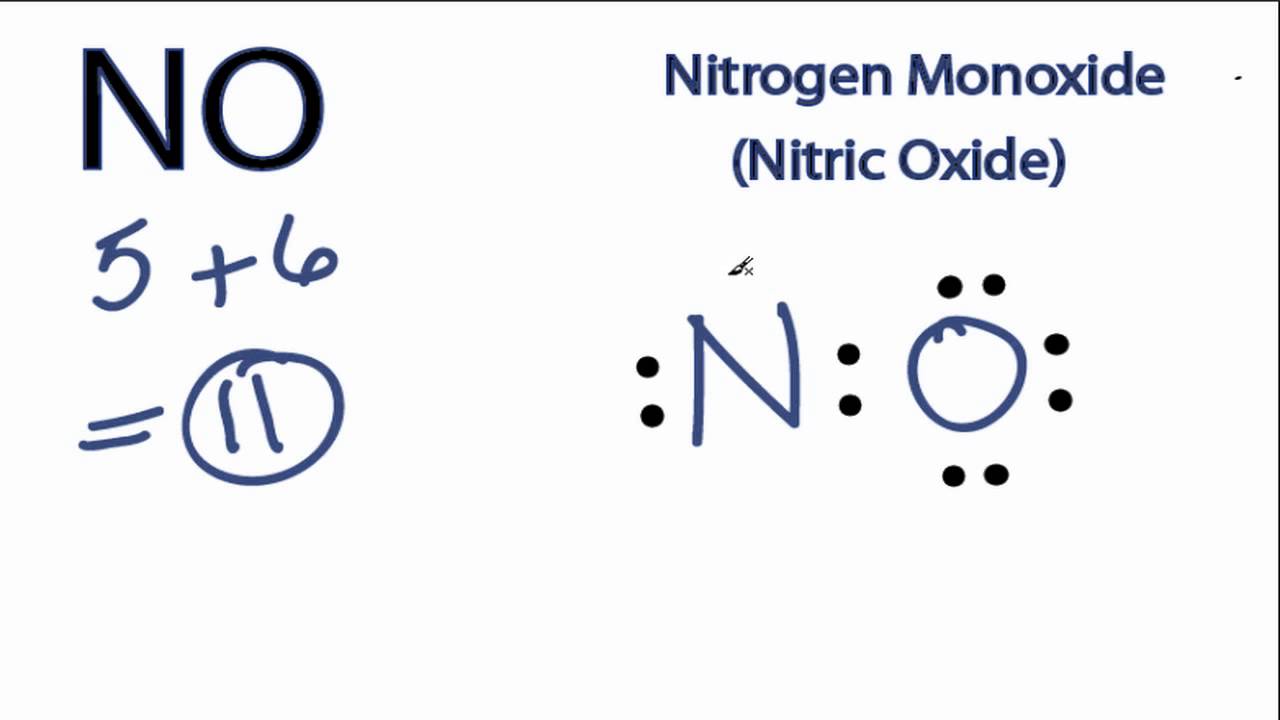
NO Lewis Structure How to Draw the Lewis Structure for NO (Nitric

How to Draw a Lewis Structure

Trick to draw Lewis dot structure for NO2 ion YouTube
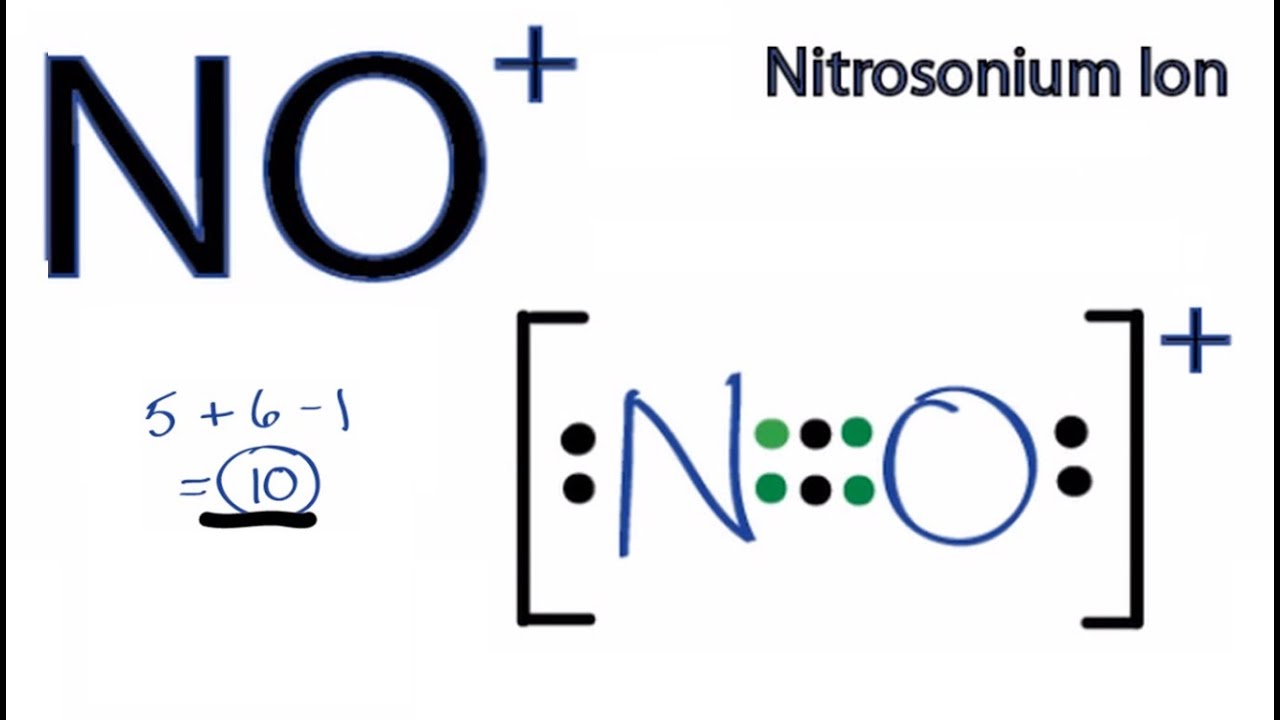
NO+ Lewis Structure How to Draw the Lewis Structure for NO+ YouTube

Drawing Lewis Structures Chemistry Socratic
Web Nitric Oxide Is A Simple Molecule Which Contains Only Two Atoms;
Web Drawing Lewis Dot Structures And Resonance Structures.
Follow These Simple Steps To Draw Lewis Dot Structures:
The Example Is For The Nitrate Ion.
Related Post: