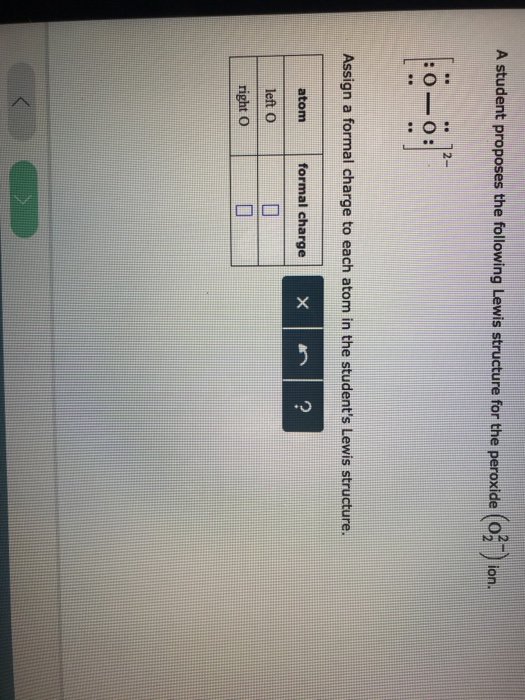
Draw The Lewis Structure For A Peroxide Ion
Draw The Lewis Structure For A Peroxide Ion - First count valence electrons for o2. Web a video explanation of how to draw the lewis dot structure for the peroxide ion, along with information about the compound including formal charges, polarity. Write lewis symbols for neutral atoms and ions. Web draw the lewis structure for a peroxide (o22−) ion. For the peroxide ion, oxygen has six valence electrons. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. For the peroxide ion, oxygen has six valence electrons. Web each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] =. Compare the bond order to that seen in the lewis structure (remember. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] =. Compare the bond order to that seen in the lewis structure (remember. And a lewis structure for −o− o−, which clearly derives from. Write lewis symbols for neutral atoms and ions. Assign a formal charge to each atom in the. A student proposes the following lewis structure for the peroxide (o2 ) ion. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Compare the bond order to that seen in the lewis structure (remember. Compare the bond order to that seen in the lewis structure (remember. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web these two electrons, according to the molecular orbital theory, complete the two π* antibonding orbitals. For the peroxide ion, oxygen has six valence electrons. Web the lewis electron structure is drawn. For the peroxide ion, oxygen has six valence electrons. Construct a qualitative molecular orbital diagram for chlorine, cl 2. Compare the bond order to that seen in the lewis structure (remember. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or. First count valence electrons for o2. For the peroxide ion, oxygen has six valence electrons. A student proposes the following lewis structure for the peroxide (o2 ) ion. Write lewis symbols for neutral atoms and ions. Web a video explanation of how to draw the lewis dot structure for the peroxide ion, along with information about the compound including formal charges, polarity. This problem has been. Web the lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! This has as result a weakening of the bond. And a lewis structure for −o− o−, which clearly derives from hydrogen peroxide, h −o −o −h, which is. Compare the bond order to that seen in the lewis structure (remember. For the peroxide ion, oxygen has six valence electrons. First count valence electrons for o2. Construct a qualitative molecular orbital diagram for chlorine, cl 2. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. This has as result a weakening of the bond strength of the peroxide. Web each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen. Compare the bond order to that seen in the lewis structure (remember. A student proposes the following lewis structure for the peroxide (o2 ) ion. Web each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 +. A student proposes the following lewis structure for the peroxide (o2 ) ion. And a lewis structure for −o− o−, which clearly derives from hydrogen peroxide, h −o −o −h, which is. This widget gets the lewis structure of chemical compounds. Web a video explanation of how to draw the lewis dot structure for the peroxide ion, along with information. Write lewis symbols for neutral atoms and ions. This widget gets the lewis structure of chemical compounds. Draw lewis structures depicting the. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. Web draw the lewis structure for a peroxide (o22−) ion. For the peroxide ion, oxygen has six valence electrons. A student proposes the following lewis structure for the peroxide (o2 ) ion. Web each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] =. Construct a qualitative molecular orbital diagram for chlorine, cl 2. Compare the bond order to that seen in the lewis structure (remember. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For the peroxide ion, oxygen has six valence electrons. Assign a formal charge to each atom in the. Web a video explanation of how to draw the lewis dot structure for the peroxide ion, along with information about the compound including formal charges, polarity. This has as result a weakening of the bond strength of the peroxide.Solved A student proposes the following Lewis structure for
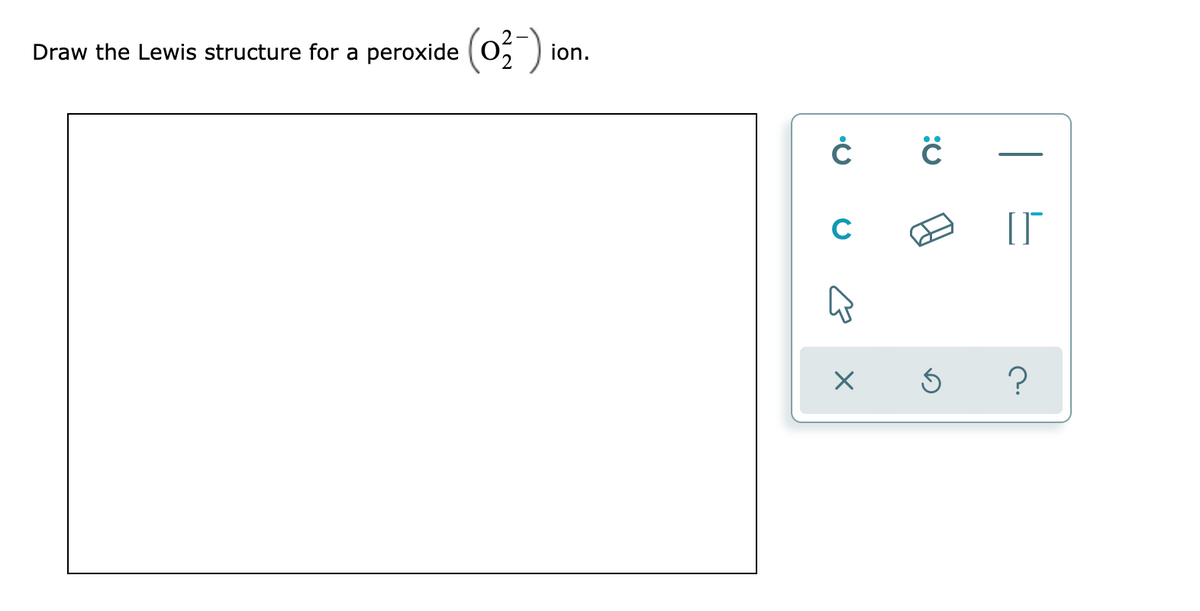
Answered Draw the Lewis structure for a peroxide… bartleby
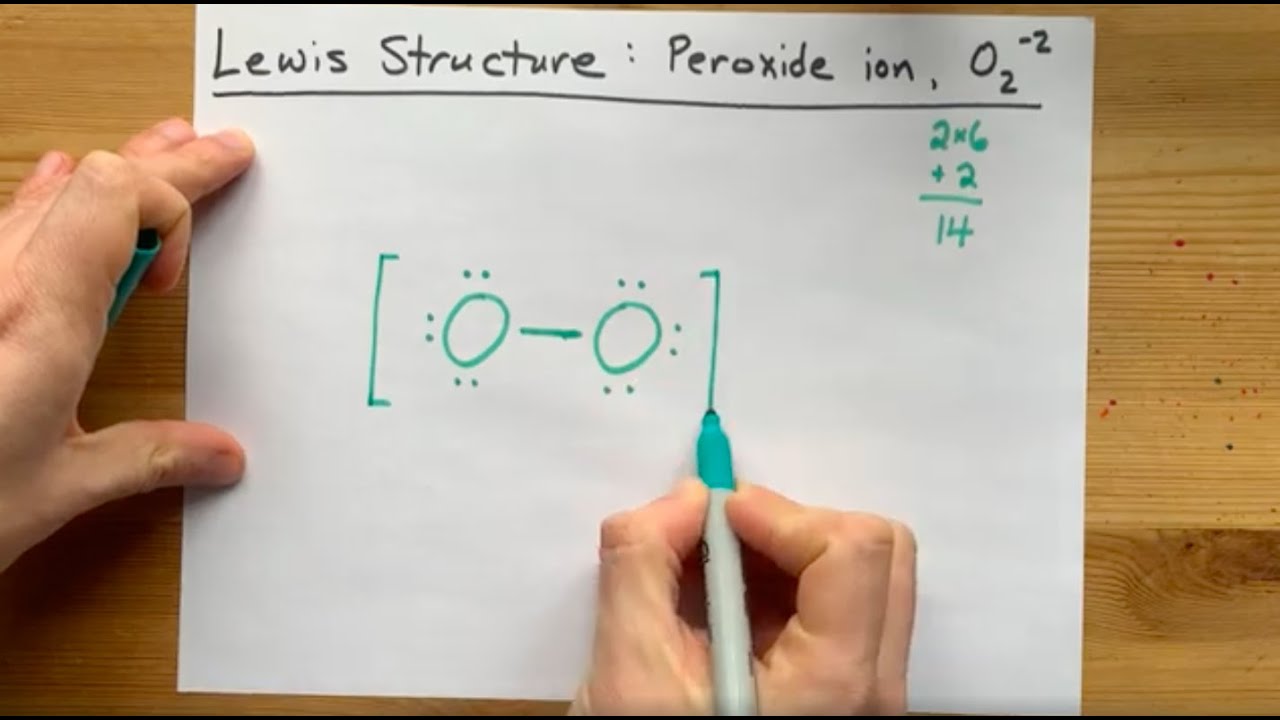
Lewis Structure of the Peroxide Ion, O2(2) YouTube
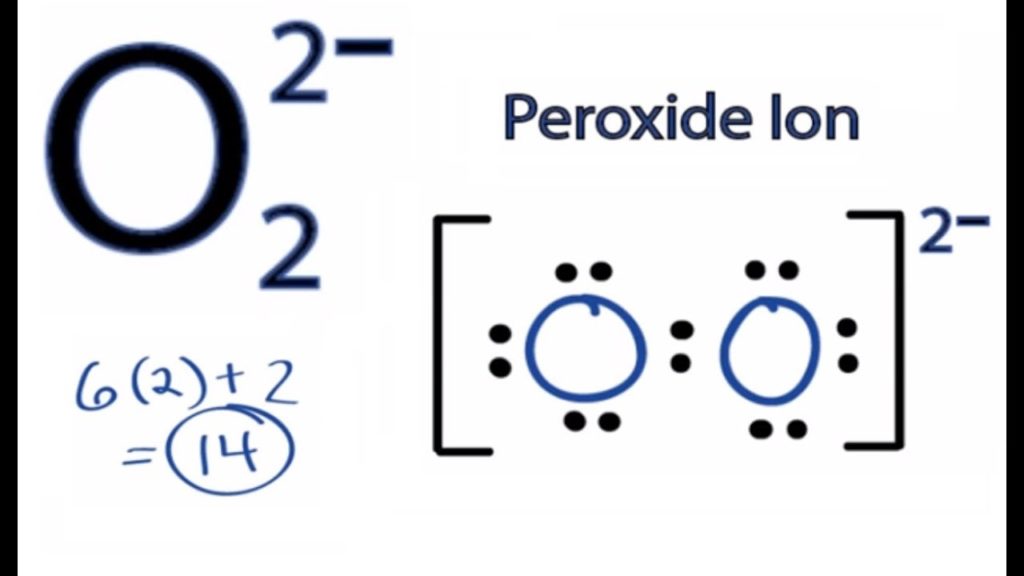
Peroxide Ion Lewis Structure

HOOH Lewis Structure How to Draw the Lewis Structure for Hydrogen

Lewis structure of O2 2 (Peroxide ion) YouTube

Lewis Structure Drawing Practice

How to make lewis structure in chem draw athomeqlero

Peroxide Ion
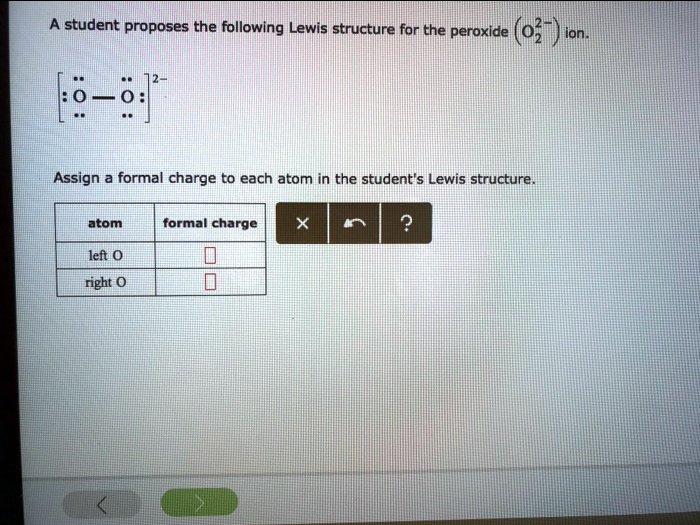
SOLVED A student proposes the following Lewis structure for the
And A Lewis Structure For −O− O−, Which Clearly Derives From Hydrogen Peroxide, H −O −O −H, Which Is.
First Count Valence Electrons For O2.
Web The Lewis Electron Structure Is Drawn Within Brackets As Is Customary For An Ion, With The Overall Charge Indicated Outside The Brackets, And The Bonding Pair Of Electrons Is.
Web These Two Electrons, According To The Molecular Orbital Theory, Complete The Two Π* Antibonding Orbitals.
Related Post: