Draw The Lewis Structure For Bf3
Draw The Lewis Structure For Bf3 - As discussed, here there are 24 electrons. Web check me out: The bf 3 lewis structure is similar to bcl 3 and bbr 3 since cl and br are in group 7 and have 7 valence electrons. Calculating the total valence electrons. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. Then, add octets to the outer atom and extra electrons to the central atom. Try to draw the bf 3 lewis structure before watching the video. Web draw the lewis structure for bf_3 in the box at the right, including lone pairs. For the bf3 structure use the periodic table to find the total number. € what is the the shape (molecular geometry) of bf3? In order to find the total valence electrons in bf3 molecule, first of all you should know the valence electrons present in boron atom as well as fluorine atom. (a) cs (b) bf4 (c) hno2 (where. What is the molecular shape of bf3? This problem has been solved! In order to draw the lewis structure of bf3, first of all you have to find the total number of valence electrons present in the bf3 molecule. Does central electron have octet? Web it is isoelectronic with carbonate anion. The central boron atom a. Calculate the total number of valence electrons. Watch the video and see if you missed any steps or information. But, as we know, there are no extra electrons. Try to draw the bf 3 lewis structure before watching the video. A prerequisite for accurately portraying the bf3 lewis structure is determining the cumulative valence electrons. Web it is isoelectronic with carbonate anion. #3 calculate and mark formal charges on the atoms, if required. Draw the lewis structure for bf3 in the window below and then answer the questions that follow. Sum the valence electrons of each atom: #1 first draw a rough sketch. Boron atom is the center atom. To accurately depict the lewis structure for bf3 (boron trifluoride) follow the below steps: (a) cs (b) bf4 (c) hno2 (where. Web drawing lewis structures for molecules with one central atom: Draw the lewis structure for boron trifluoride (bf 3). Boron atom is the center atom. Web draw the lewis structure for bf_3 in the box at the right, including lone pairs. #1 first draw a rough sketch. For the central boron atom: Add octets to outer atoms: Draw the lewis structure for bf3 in the window below and then answer the questions that follow. Web check me out: Drawing lewis structures for bf3, pf3 and brf3; But, as we know, there are no extra electrons. Sum the valence electrons of each atom: Does central electron have octet? Starting from this structure, complete the lewis structure that follows the octet rule on all atoms. Web use these steps to correctly draw the bf 3 lewis structure: Web drawing the lewis structure for bf 3. Find the total valence electrons in bf3 molecule. € what is the the shape (molecular geometry) of bf3? What is the molecular shape of bf3? Starting from this structure, complete the lewis structure that follows the octet rule on all atoms. (valence electrons are the number of electrons present in the outermost shell of an atom). Web how to draw lewis structure for bf3 boron trifluoride?lewis structure: First, determine the total number of valence electrons. What is the molecular shape of bf_3? Try to draw the bf 3 lewis structure before watching the video. Web check me out: This shape makes an equilateral triangle with each side making a. Boron (b) doesn't need 8 valence electrons to have an octet (boron often only needs 6). Sum the valence electrons of each atom: The central boron atom a. A prerequisite for accurately portraying the bf3 lewis structure is determining the cumulative valence electrons. Does central electron have octet? Using formal charges to determine how many bonds to make, a different perspective. Find the total valence electrons in bf3 molecule. For the central boron atom: Try structures similar to bf 3 for more practice. Let’s discuss each step in more detail. Draw a lewis structure for each of the following molecules or ions: Web drawing the lewis structure for bf 3. #1 first draw a rough sketch. #3 calculate and mark formal charges on the atoms, if required. This shape makes an equilateral triangle with each side making a. (a) cs (b) bf4 (c) hno2 (where. (valence electrons are the number of electrons present in the outermost shell of an atom).
How to Draw the Lewis Dot Structure for BF3 Boron trifluoride YouTube

BF3 Lewis Structure (Boron Trifluoride) YouTube

BF3 lewis structure, molecular geometry, hybridization, bond angle

Bf3 Lewis Structure Lone Pairs Draw Easy

BF3 Lewis Structure How to Draw the Lewis Structure for BF3 YouTube
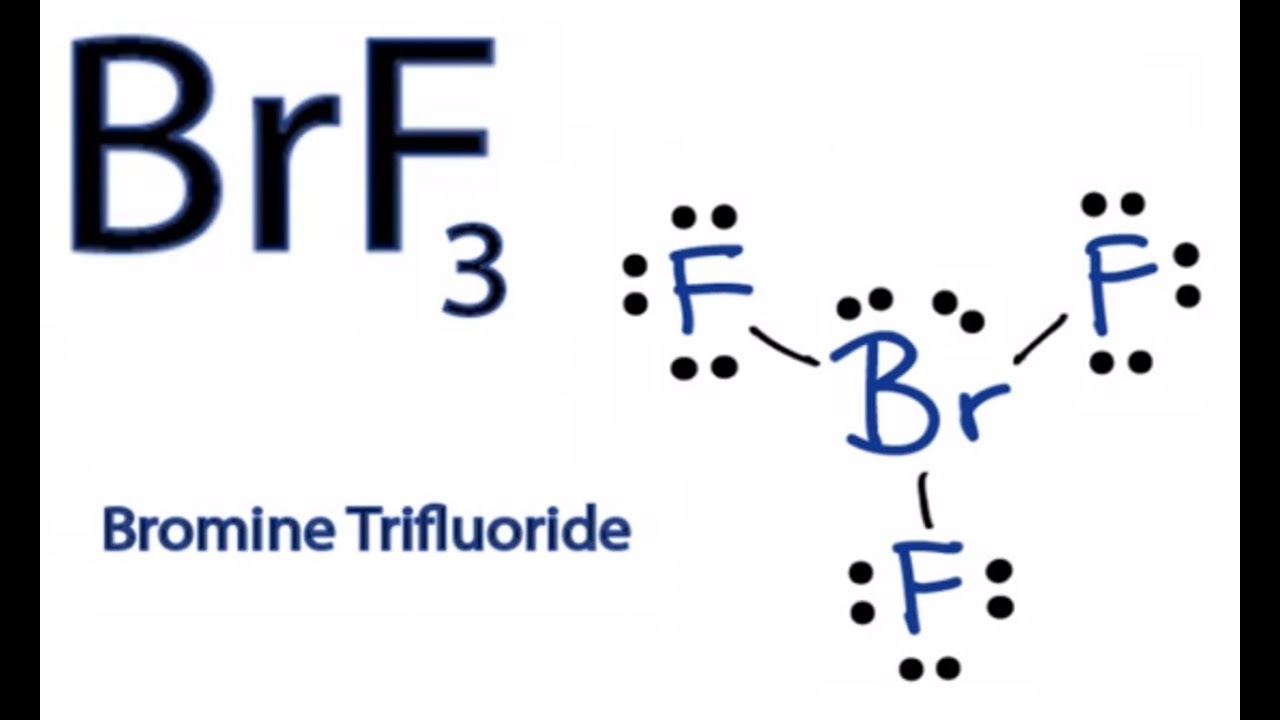
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube

BF3 Lewis Structure YouTube
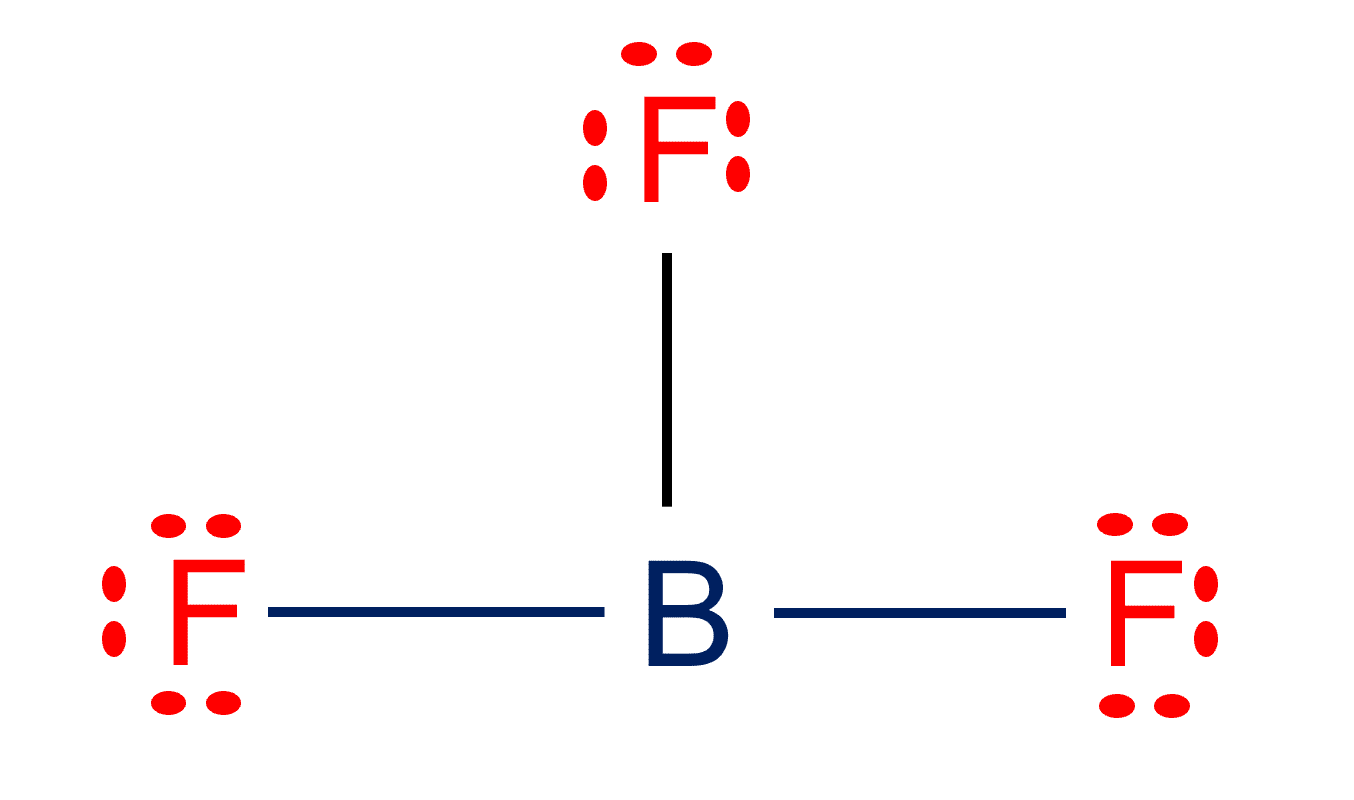
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity

BF3 lewis structure and Molecular Geometry YouTube

Bf3 Lewis Structure Molecular Geometry And Hybridization itechguides
#2 Mark Lone Pairs On The Atoms.
What Is The Molecular Shape Of Bf_3?
Add Electrons (3*7) + 3 = 24.
Draw The Lewis Structure For Boron Trifluoride (Bf 3).
Related Post: