Draw The Lewis Structure For The Azide Ion
Draw The Lewis Structure For The Azide Ion - It is a chemical formula for the azide ion. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web 70 more lewis dot structures. You'll get a detailed solution from a subject matter. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. See how the three nitrogen atoms are bonded in this interactive 3d model. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. It has 5 valence electrons. Chemistry covalent bonds drawing lewis structures. I also go over hybridization, shape and bond angles. Web the lewis structure of the azide ion, [n₃]−, can be drawn by placing the three nitrogen atoms on the grid and connecting them with double bonds. This problem has been solved! The central n atom carries a negative charge. Sodium azide is found in air bags of automobiles. See how the three nitrogen atoms are bonded in this interactive. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. The ion is made up of three nitrogen. Web explore the formula and structure of the azide ion, a common component of explosives and propellants. See how the three nitrogen atoms are bonded in this interactive 3d model.. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. The azide ion is a. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. Sodium azide is found in air bags of automobiles. Azide does have resonance structures. Web 70 more lewis dot structures. Draw the lewis structure for the azide (n3) ion. You'll get a detailed solution from a subject matter. N3 lewis structure, hybridization, molecular structure, bond angle and shape. Determine the total number of valence. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Nitrogen ordinarily has five electrons in its valence; This problem has been solved! Determine the total number of valence. Nitrogen is in group 5, also called 15, on the periodic table. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. Web the lewis structure of the azide ion, [n₃]−, can be drawn by placing the three nitrogen atoms on the grid and connecting them with double bonds. The vsepr model predicts a shape that is linear. You'll get a detailed solution. Sodium azide is found in air bags of automobiles. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. N3 lewis structure, hybridization, molecular structure, bond angle and shape. It has 5 valence electrons. Web what is the lewis structure for the most stable azide ion, n − 3? Sodium azide is found in air bags of automobiles. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. See how the three nitrogen atoms are bonded in this interactive 3d model. Web when forming a lewis symbol for an ion, the chemical symbol is surrounded by dots. Nitrogen ordinarily has five electrons in its valence; Sodium azide is found in air bags of automobiles. The ion is made up of three nitrogen. Web when forming a lewis symbol for an ion, the chemical symbol is surrounded by dots that are used to represent valence electrons, and the whole structure is placed in square. The azide ion is. N3 lewis structure, hybridization, molecular structure, bond angle and shape. Draw the lewis structure for the azide (n3) ion. Nitrogen ordinarily has five electrons in its valence; The ion is made up of three nitrogen. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web explore the formula and structure of the azide ion, a common component of explosives and propellants. It has 5 valence electrons. Sodium azide is found in air bags of automobiles. The azide ion is a. It is a chemical formula for the azide ion. I also go over hybridization, shape and bond angles. Chemistry covalent bonds drawing lewis structures. Web what is the lewis structure for the most stable azide ion, n − 3? You'll get a detailed solution from a subject matter. The ion is made up of three nitrogen. The central n atom carries a negative charge. Nitrogen ordinarily has five electrons in its valence; N3 lewis structure, hybridization, molecular structure, bond angle and shape. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. Web the lewis structure of the azide ion, [n₃]−, can be drawn by placing the three nitrogen atoms on the grid and connecting them with double bonds. Web when forming a lewis symbol for an ion, the chemical symbol is surrounded by dots that are used to represent valence electrons, and the whole structure is placed in square.
N3 (Azide Ion) Molecular Geometry, Bond Angles & Electron Geometry
Solved Draw the Lewis structure for the azide (N3) ion.

Day03 4 Azide Ion (N3) & N2O4 Resonance & Avg Bond Order YouTube

Molecular Orbitals Azide Anion
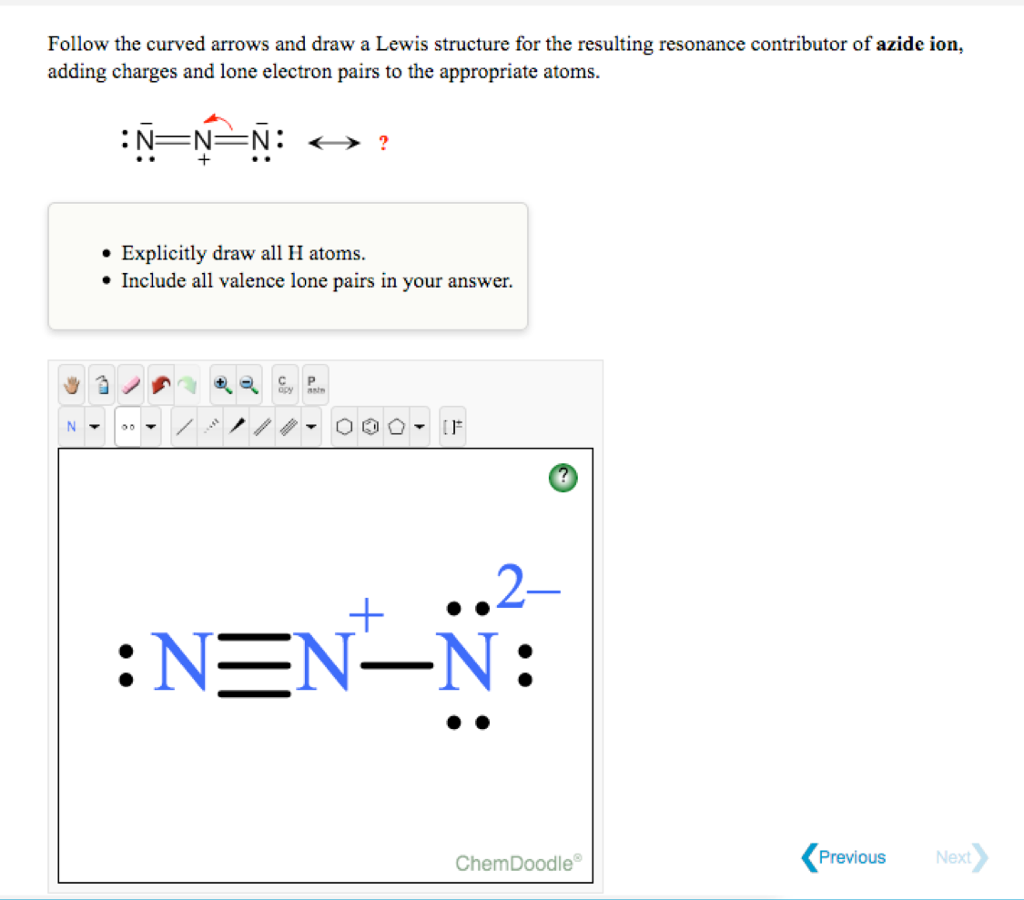
Solved Follow the curved arrows and draw a Lewis structure

Lewis Structure for N3 Azide ion YouTube
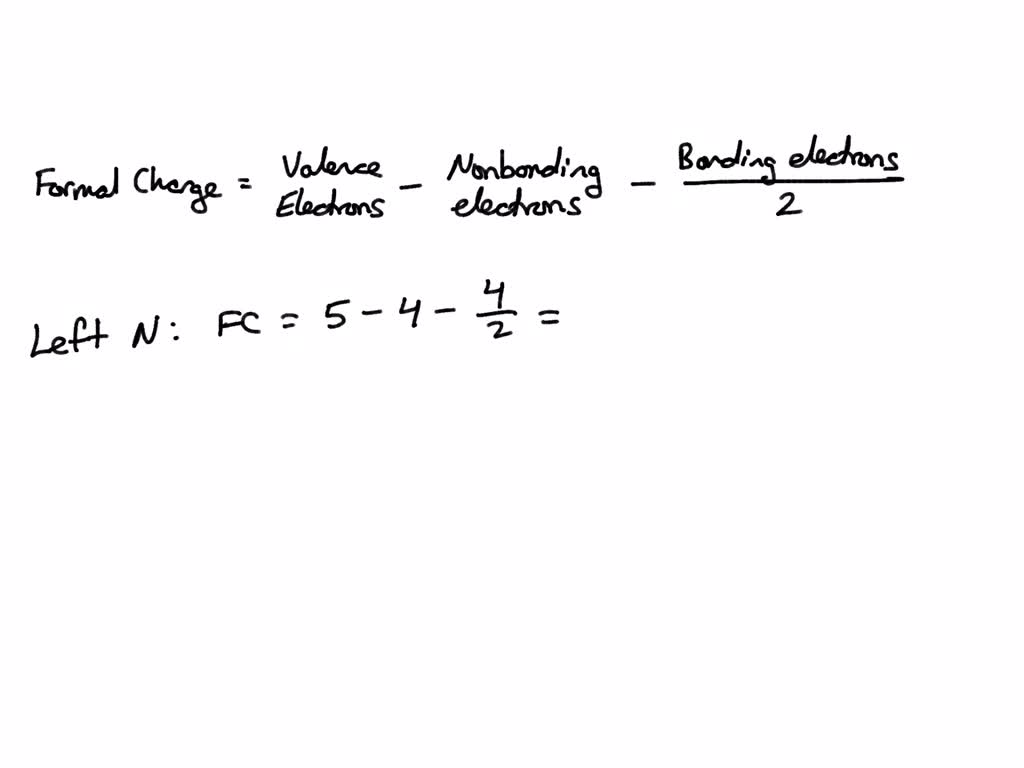
SOLVED Draw the Lewis structure of the azide ion, N3, and calculate
Solved Draw the Lewis structure for the azide (N, ion. Ć n

Draw the structure of azide ion \( \mathrm{P} \) YouTube
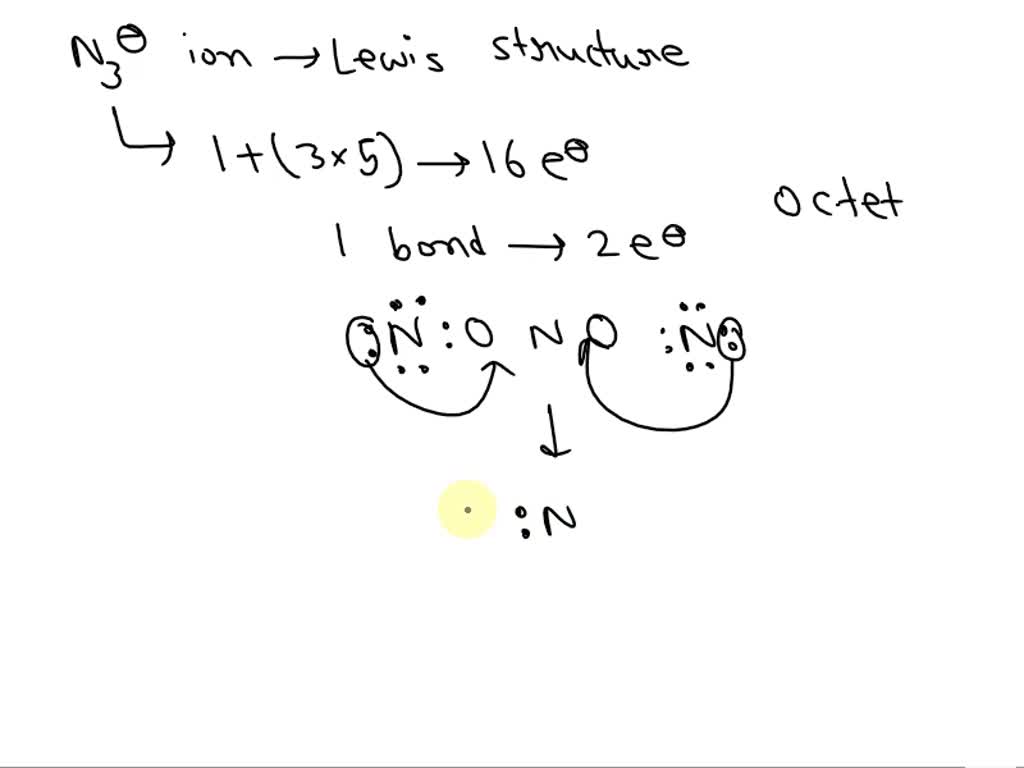
SOLVED Draw the Lewis structure of azide ion, Ns. (6 pts.)
Draw The Lewis Structure For The Azide (N3) Ion.
The Vsepr Model Predicts A Shape That Is Linear.
See How The Three Nitrogen Atoms Are Bonded In This Interactive 3D Model.
Nitrogen Is In Group 5, Also Called 15, On The Periodic Table.
Related Post: