Draw The Lewis Structure Of Bf3
Draw The Lewis Structure Of Bf3 - Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Web the lewis structure for ammonia (nh₃) shown below is incorrect. O 180 o 109.5 o $109.5 o 120 In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. Web boron trifluoride contains one boron atom and three fluorine atoms. Web in this video, we are going to draw the lewis structure for bf₃, boron trifluoride. For bf3, the total count of valence electrons is 24. For the bf 3 lewis structure, calculate the total number of valence electrons for the bf 3 molecule. Web how to draw the bf3 lewis structure. Web here’s the best way to solve it. In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. Draw the lewis structure for bf_3 in the box at the right, including lone pairs. To accurately depict the lewis structure for bf3 (boron trifluoride) follow the below steps: In order to find the total valence electrons in bf3 molecule, first of. Lewis structure of boron trifluoride (bf 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. So, the lewis structure of bf3. For the central boron atom: State the hybridization of the central atom of. The bf 3 lewis structure is similar to bcl 3 and bbr 3 since cl and br are in group 7 and have 7 valence electrons. But, as we know, there are no extra electrons. Video answers to similar questions. Draw (on paper) a lewis structure for bf3 and answer the following questions based on your drawing. Web check me out: The central boron atom a. Web steps of drawing bf3 lewis structure step 1: For the bf 3 lewis structure, calculate the total number of valence electrons for the bf 3 molecule. So, the lewis structure of bf3 is: Draw a lewis structure for each of the following molecules or ions: Draw the lewis structure for bf_3 in the box at the right, including lone pairs. State the hybridization of the central atom of. In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. Draw a lewis structure for each of the following molecules or ions: Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. O 180 o 109.5 o $109.5 o 120 The final answer must have this. We will be doing this in 5 simple steps. To draw a lewis structure, first of all, add electrons and draw the connectivities. For the central boron atom: The central boron atom a. Use these steps to correctly draw the bf 3 lewis structure: The central boron atom a. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Web a video explanation of how to draw the lewis dot structure for boron trifluoride, along with information about the compound including formal charges, polarit. Web. The total number of valence electrons. What is the molecular shape of bf_3? O 180 o 109.5 o $109.5 o 120 Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. If you're not sure you have the best lewis structure. Web the lewis structure for ammonia (nh₃) shown below is incorrect. What is the molecular shape of bf3? Calculate the total number of valence electrons. So, the lewis structure of bf3 is: Draw the lewis structure for bf_3 in the box at the right, including lone pairs. If you're not sure you have the best lewis structure for bf 3 you can calculate the formal charges. Web the lewis structure for ammonia (nh₃) shown below is incorrect. So, the lewis structure of bf3 is: Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Drawing the lewis structure for bf 3. Web bf3 draw the lewis structure for bf3 in the box at the right, including lone pairs. What is the molecular shape of bf3? As discussed, here there are 24 electrons. For the central boron atom: Web how to draw the bf3 lewis structure. Web 5 steps to draw the lewis structure of bf3 step #1: Boron (b) doesn't need 8 valence electrons to have an octet (boron often only needs 6). Add electrons (3*7) + 3 = 24. Draw the lewis structure for boron trifluoride (bf 3). For bf3, the total count of valence electrons is 24. Starting from this structure, complete the lewis structure that follows the octet rule on all atoms.
BF3 Lewis Structure, Molecular Geometry, and Hybridization

How to Draw the Lewis Dot Structure for BF3 Boron trifluoride YouTube
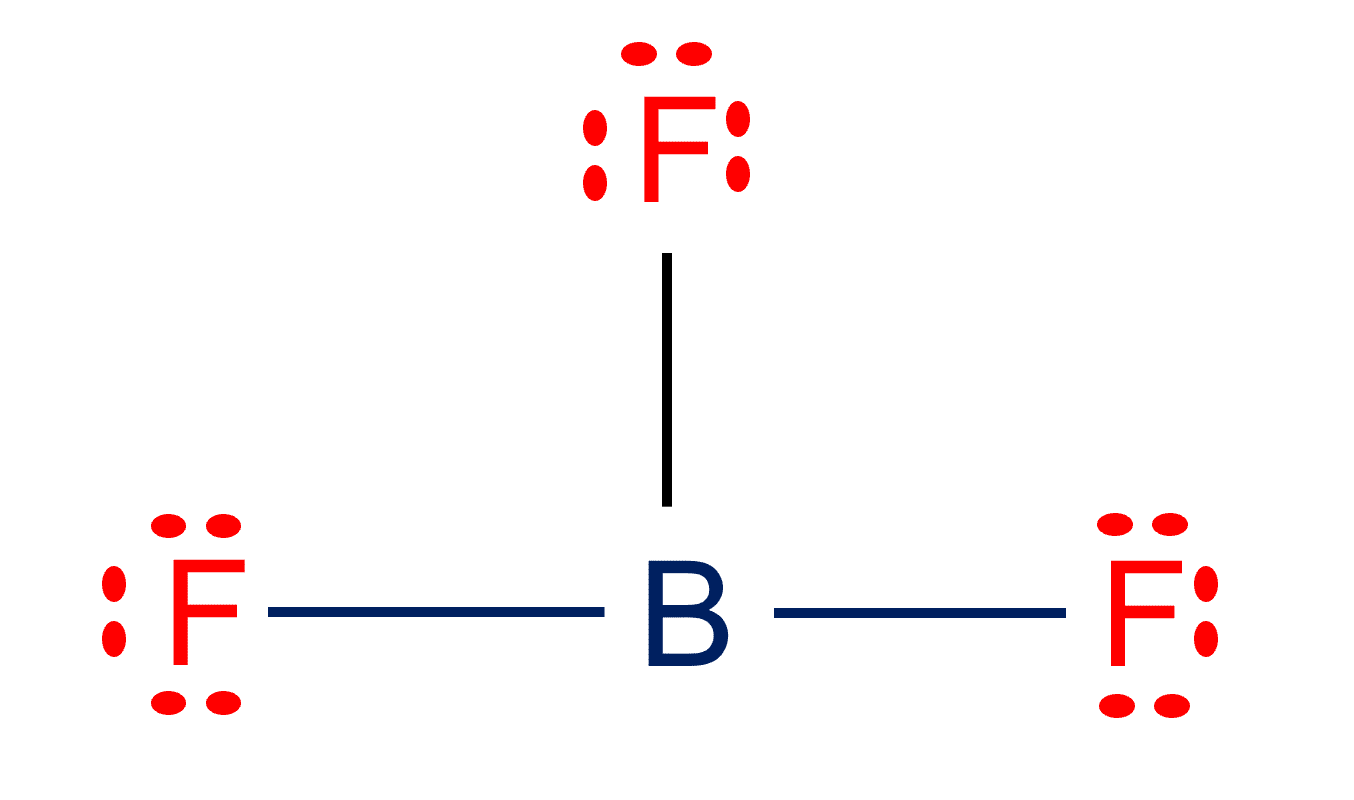
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity

BF3 Lewis Structure (Boron Trifluoride) YouTube
![Molecular Geometry of BF3 [with video and free study guide]](https://aceorganicchem.com/chemistry/wp-content/uploads/2023/05/BF3-lewis-dot--1024x539.jpg)
Molecular Geometry of BF3 [with video and free study guide]

BF3 lewis structure and Molecular Geometry YouTube

BF3 lewis structure, molecular geometry, hybridization, bond angle
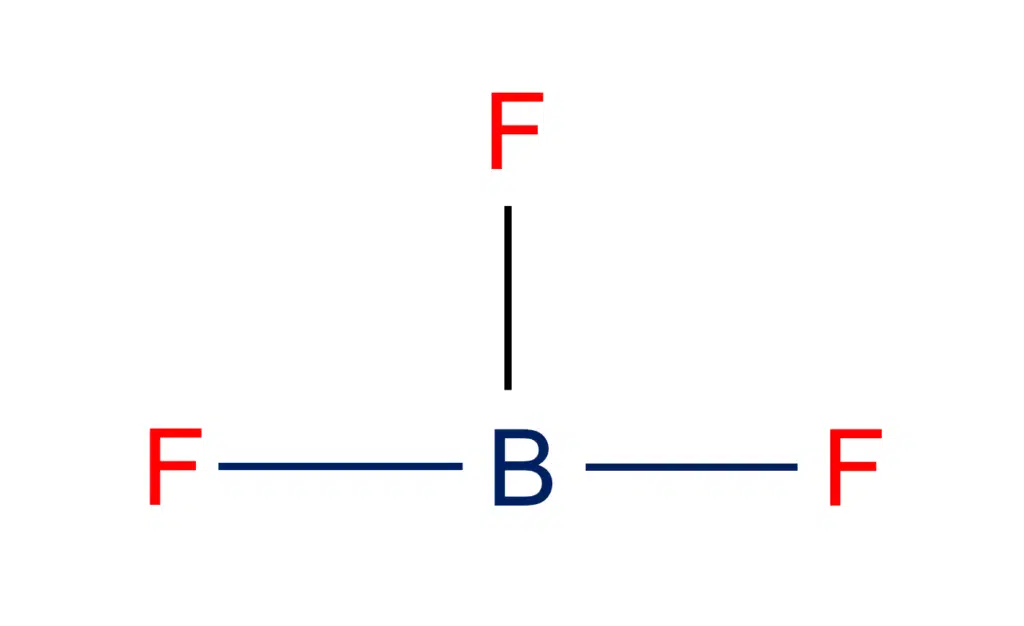
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity

BF3 Lewis Structure How to Draw the Lewis Structure for BF3 YouTube

Bf3 Lewis Structure Lone Pairs Draw Easy
We Will Be Doing This In 5 Simple Steps.
To Learn About Any Lewis Dot Structure Of Boron Trifluoride Bf3, You Need To Compute Mainly Four Important Things.
Web Drawing The Lewis Structure For Bf 3.
The Bf 3 Lewis Structure Is Similar To Bcl 3 And Bbr 3 Since Cl And Br Are In Group 7 And Have 7 Valence Electrons.
Related Post: