Draw The Lewis Structure Of Cof2 Include Lone Pairs
Draw The Lewis Structure Of Cof2 Include Lone Pairs - Match the words in the left column to the appropriate blanks in the sentences on the right. Web in the lewis structure for cof 2 there are a total of 24 valence electrons. Web the electron pair being shared by the atoms is called a bonding pair ; The other three pairs of electrons on each chlorine atom are called lone pairs. There are 2 steps to solve this one. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Draw the lewis structure of cof2. Web include all lone pairs of electrons. Part a for cof2 draw an appropriate lewis structure (carbon is the central atom). Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. In order to find the total valence electrons in a. Draw the lewis structures of the following molecules. Since the lone pair electrons are often not shown in chemical. Web june 12, 2022 by aditi roy. 3 attempts left check my work be sure to answer all parts. Web draw a skeleton structure of the molecule. In turn, each oxygen atom is bonded to one of the fluorine atoms. Include lone pairs on all atoms, where appropriate. If the central atom has fewer electrons than an octet, use. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. To properly draw the cof 2 lewis structure, follow these steps: In this structure, carbon is the central atom. Web in the lewis structure for cof 2 there are a total of 24 valence electrons. Draw the molecule by placing atoms on the grid. Draw the lewis structure of cof2. Since the lone pair electrons are often not shown in chemical. The other three pairs of electrons on each chlorine atom are called lone pairs. Web the electron pair being shared by the atoms is called a bonding pair ; {a;} submitted by lauren m. Determine the geometry of cof2 using vsepr theory, and then identify whether the molecule is. The carbon atom is the central atom and is bonded to the two oxygen atoms. {a;} submitted by lauren m. To properly draw the cof 2 lewis structure, follow these steps: There are 2 steps to solve this one. Part a for cof2 draw an appropriate lewis structure (carbon is the central atom). Lone pairs are not involved in. The other three pairs of electrons on each chlorine atom are called lone pairs. There are 2 steps to solve this one. To properly draw the cof 2 lewis structure, follow these steps: Select draw rings more f o с. Determine the geometry of cof2 using vsepr theory, and then identify whether the molecule is polar or nonpolar. There are 2 steps to solve this one. Web draw a skeleton structure of the molecule. If the central atom has fewer electrons than an octet, use. Web the lewis structure indicates that each cl atom has three pairs of electrons that. Include lone pairs on all atoms, where appropriate. .the lewis structure of cof2 consists of one carbon atom, two oxygen atoms, and two fluorine atoms. The carbon atom is the central atom and is bonded to the two oxygen atoms. Web the lewis structure of cof2 is c: Find the total valence electrons in cof2 molecule. In this structure, carbon is the central atom. Web the lewis structure of cof2 is c: Web in the lewis structure for cof 2 there are a total of 24 valence electrons. You'll need to form a double bond between the carbon and oxygen to complete the octet on the. Web draw the lewis structure of the following molecule. Web in the lewis structure for cof 2 there are a total of 24 valence electrons. If the central atom has fewer electrons than an octet, use. Determine the geometry of cof2 using vsepr theory, and then identify whether the molecule is polar or nonpolar. Since the lone pair electrons are often not shown in chemical. Web adding the remaining. Match the words in the left column to the appropriate blanks in the sentences on the right. Lone pairs are not involved in. To properly draw the cof 2 lewis structure, follow these steps: Web the electron pair being shared by the atoms is called a bonding pair ; The carbon atom is the central atom and is bonded to the two oxygen atoms. Draw a lewis structure for the following. Web adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure: Web draw the lewis structure of the following molecule. 3 attempts left check my work be sure to answer all parts. Draw the molecule by placing atoms on the grid. In order to find the total valence electrons in a. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3. Web in the lewis structure for cof 2 there are a total of 24 valence electrons. Draw the lewis structures of the following molecules. Select draw rings more f o с. In turn, each oxygen atom is bonded to one of the fluorine atoms.
Lewis Dot Structure For C2f2
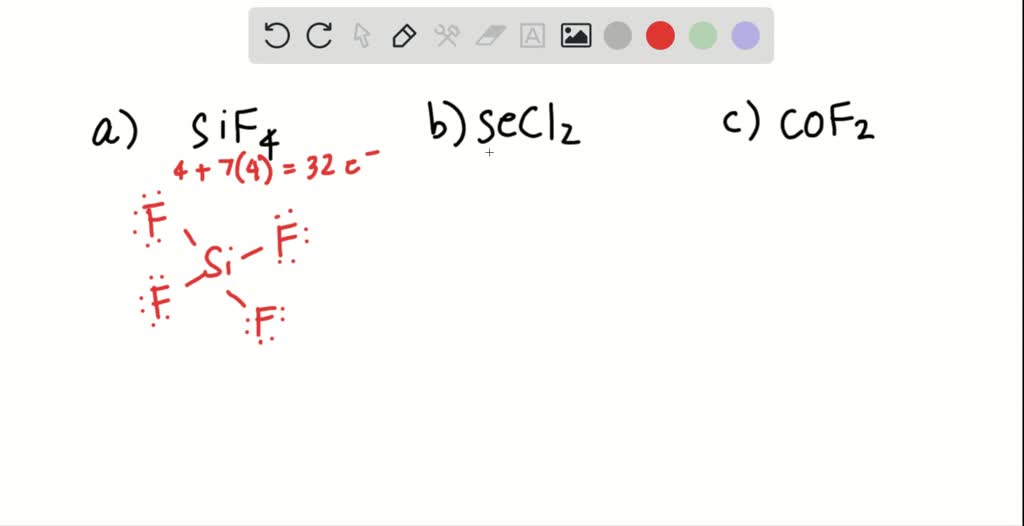
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is

Lewis Structure Of Cof2
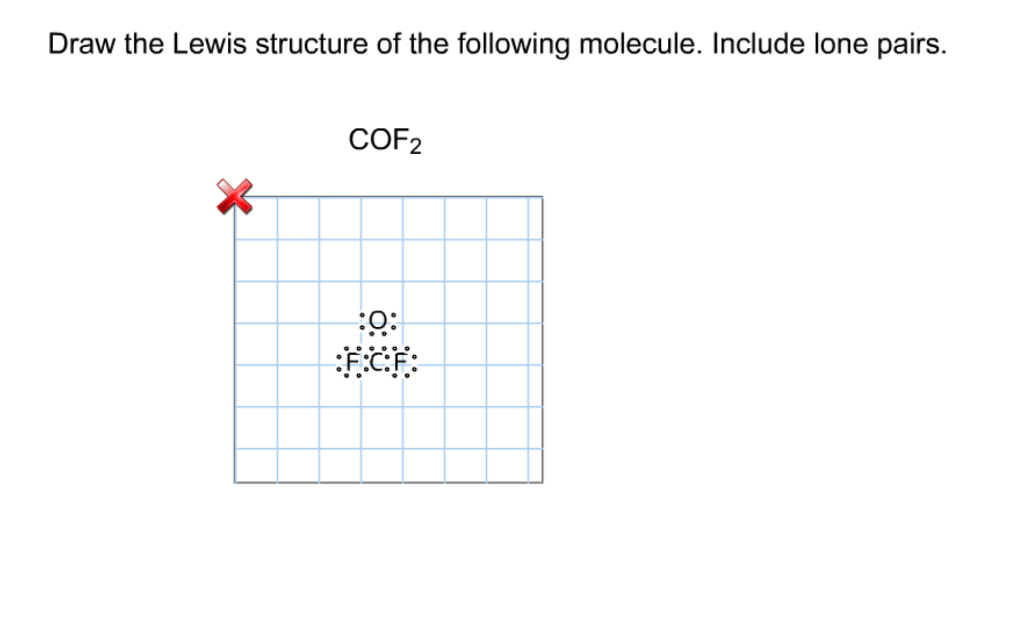
Solved Draw the Lewis structure of the following molecule.

Draw the Lewis structure (including all lone pair electrons and any

Draw the Lewis structure for carbonyl fluoride, COF2 What are the

How To Draw Lewis Structures A Step By Step Tutorial

Draw The Lewis Structure Of COF2. Include Lone Pairs.
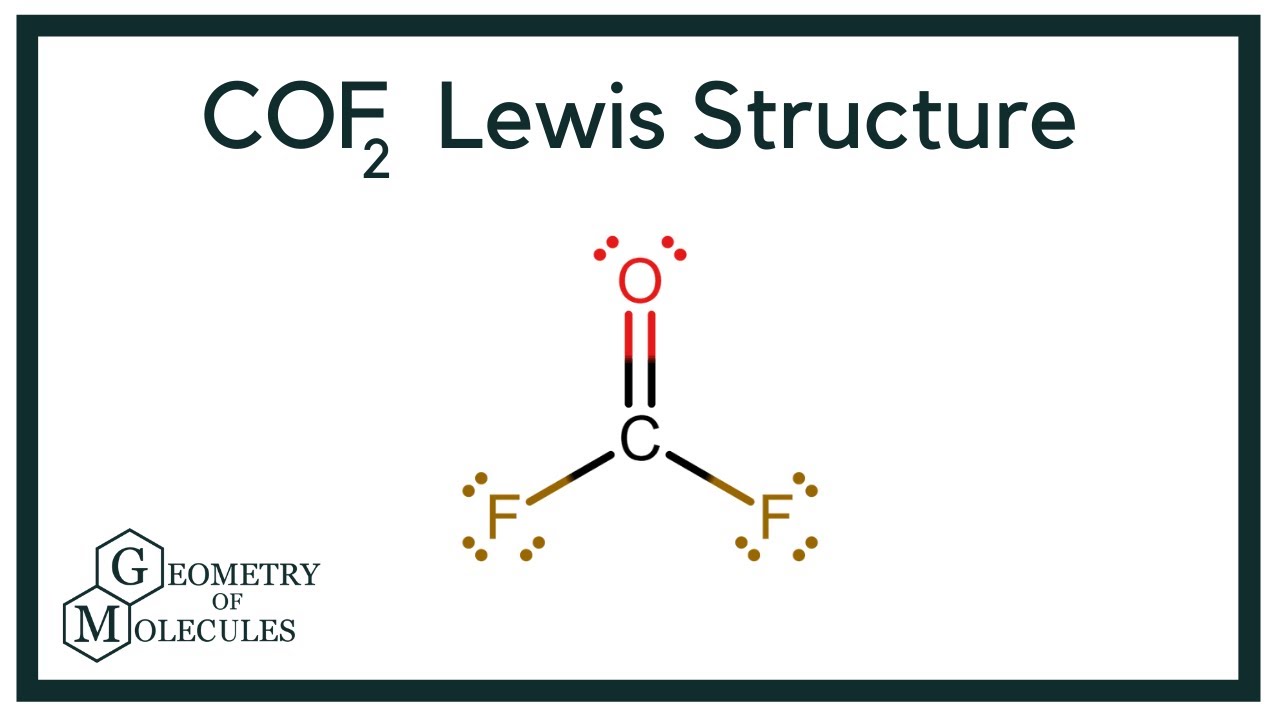
COF2 Lewis Structure (Carbonyl Fluoride) YouTube

COF2 Lewis Structure How to Draw the Lewis Structure for COF2 YouTube
Web Include All Lone Pairs Of Electrons.
Web The Lewis Structure Indicates That Each Cl Atom Has Three Pairs Of Electrons That Are Not Used In Bonding (Called Lone Pairs) And One Shared Pair Of Electrons (Written Between.
There Are 2 Steps To Solve This One.
The Cof2 Lewis Structure Refers To The Arrangement Of Atoms And Electrons In Carbon Dioxide Difluoride.
Related Post: