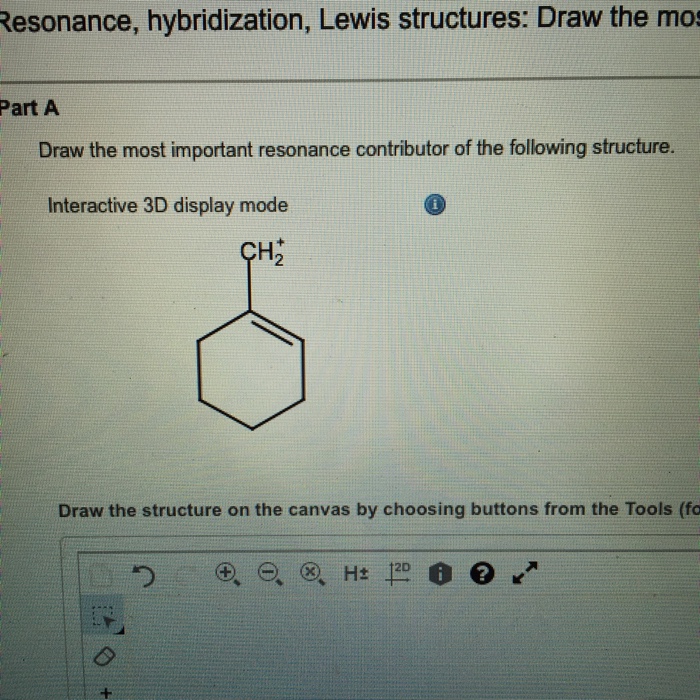
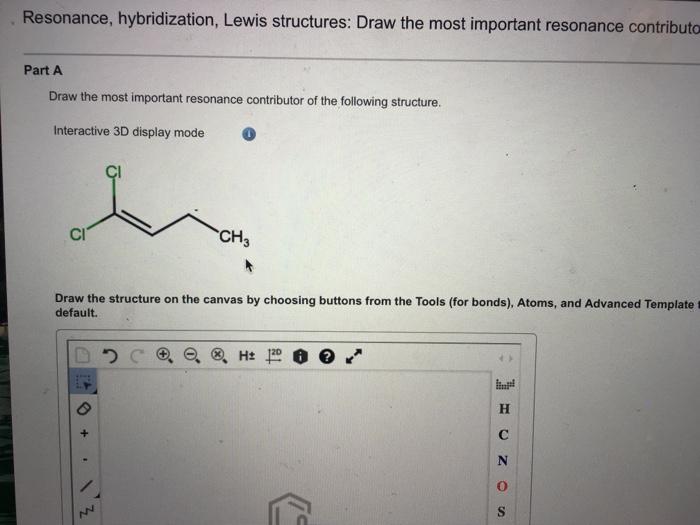
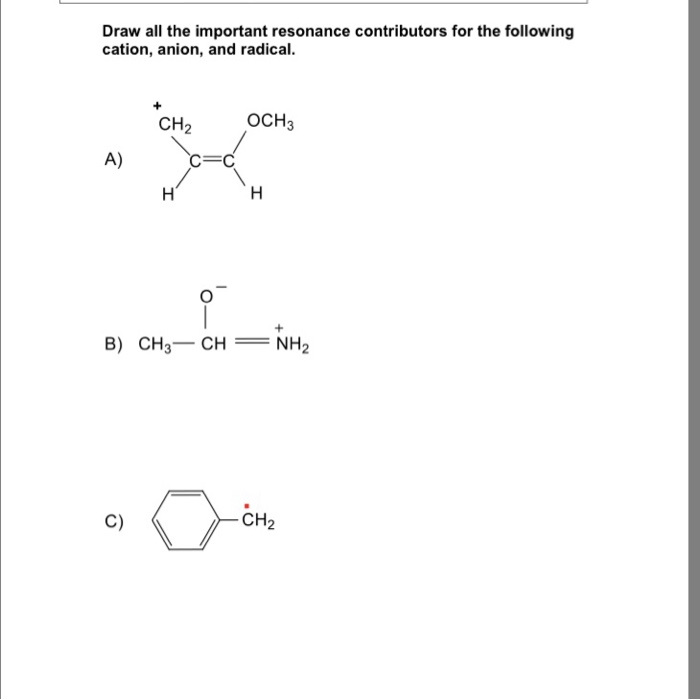
Draw The Most Important Resonance Contributor Of The Following Structure
Draw The Most Important Resonance Contributor Of The Following Structure - Interactive 3d display mode ch h.ch draw the. This is important because neither resonance structure. Resonance structures are various forms of. Oxygen atoms (3*6) = 18. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Begin by watching this video: Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an. The arrangement of atoms in a molecule or ion is called its. Draw the most important resonance contributor of. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. This is important because neither resonance structure. Begin by watching this video: Draw resonance forms and predict the relative contribution of each. Resonance structures are various forms of. Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. Draw the most important resonance contributor of the following structure. Draw the most important resonance contributor of the following species. Resonance structures for acetate ion. Draw the most important resonance contributor of the following structure. The arrangement of atoms in a molecule or ion is called its. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Resonance structures are various forms of. Resonance contributors and the resonance hybrid. Resonance structures are various forms of. Draw the most important resonance contributor of. Web draw the resonance structures for benzene. Draw resonance forms and predict the relative contribution of each. Interactive 3d display mode draw the structure. The arrangement of atoms in a molecule or ion is called its. Resonance contributors and the resonance hybrid. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Draw the most important resonance contributor of the following species. The most significant resonance contributor has the greatest number of full octets (or if applicable,. The arrangement of atoms in a molecule or ion is called its. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: Web draw the resonance structures for benzene. Draw the most important resonance contributor of the following species. 1) when you see two different resonance. Draw the most important resonance contributor of the following species. Web draw the resonance structures for benzene. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Draw the most important resonance contributor of the following structure. Web rules for drawing resonance structures: Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Interactive 3d display mode. Draw the most important resonance contributor of the following species. The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets ). Web rules for drawing resonance structures: Begin by watching this video: Oxygen atoms (3*6) = 18. Interactive 3d display mode ch h.ch draw the. Oxygen atoms (3*6) = 18. Interactive 3d display mode draw the structure. The arrangement of atoms in a molecule or ion is called its. Draw resonance forms and predict the relative contribution of each. Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. Web rules for drawing resonance structures: Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an. Interactive 3d display mode ch h.ch draw the. Calculate the total number of valence electrons from each atom. Draw the most important resonance contributor of. Begin by watching this video: Resonance structures for acetate ion. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets ). Oxygen atoms (3*6) = 18. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Draw the most important resonance contributor of the following species. Web draw the resonance structures for benzene. Draw resonance forms and predict the relative contribution of each.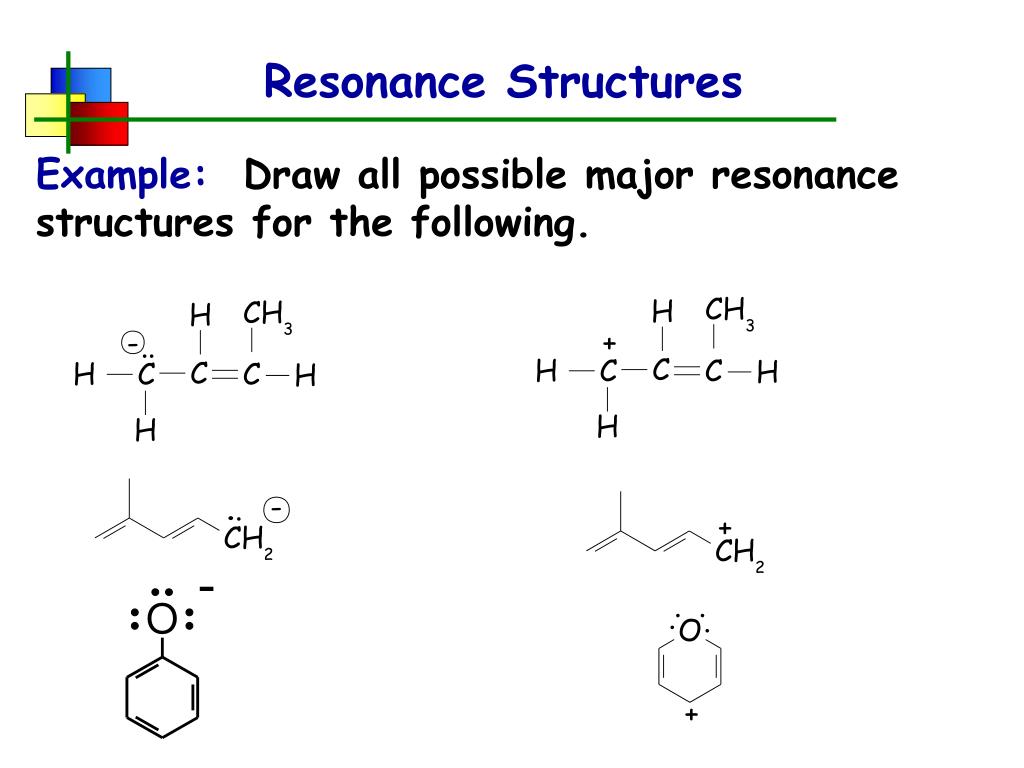
PPT Resonance Structures PowerPoint Presentation, free download ID
Solved Draw the most important resonance contributor of the

draw significant resonance structures for the following compound
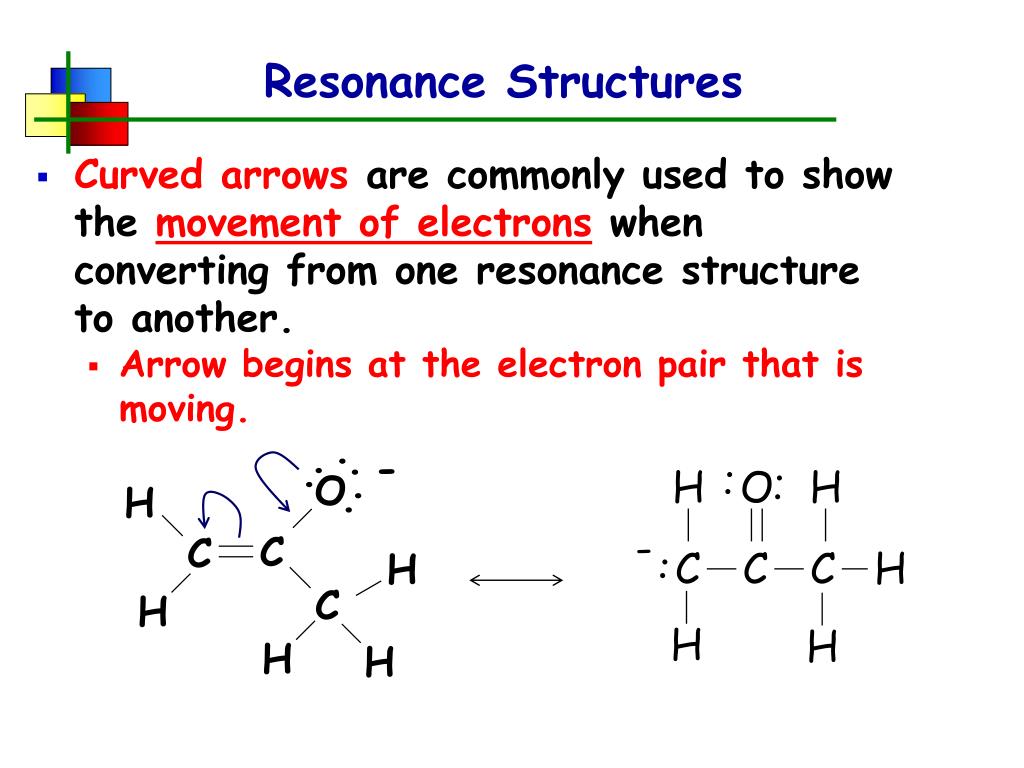
PPT Resonance Structures PowerPoint Presentation, free download ID
Solved Draw the most important resonance contributor of the
Solved Draw the most important resonance contributor for the

How to Draw Resonance Contributors MCC Organic Chemistry
Solved Draw all the important resonance contributors for the

Solved Draw the most important resonance contributor of the
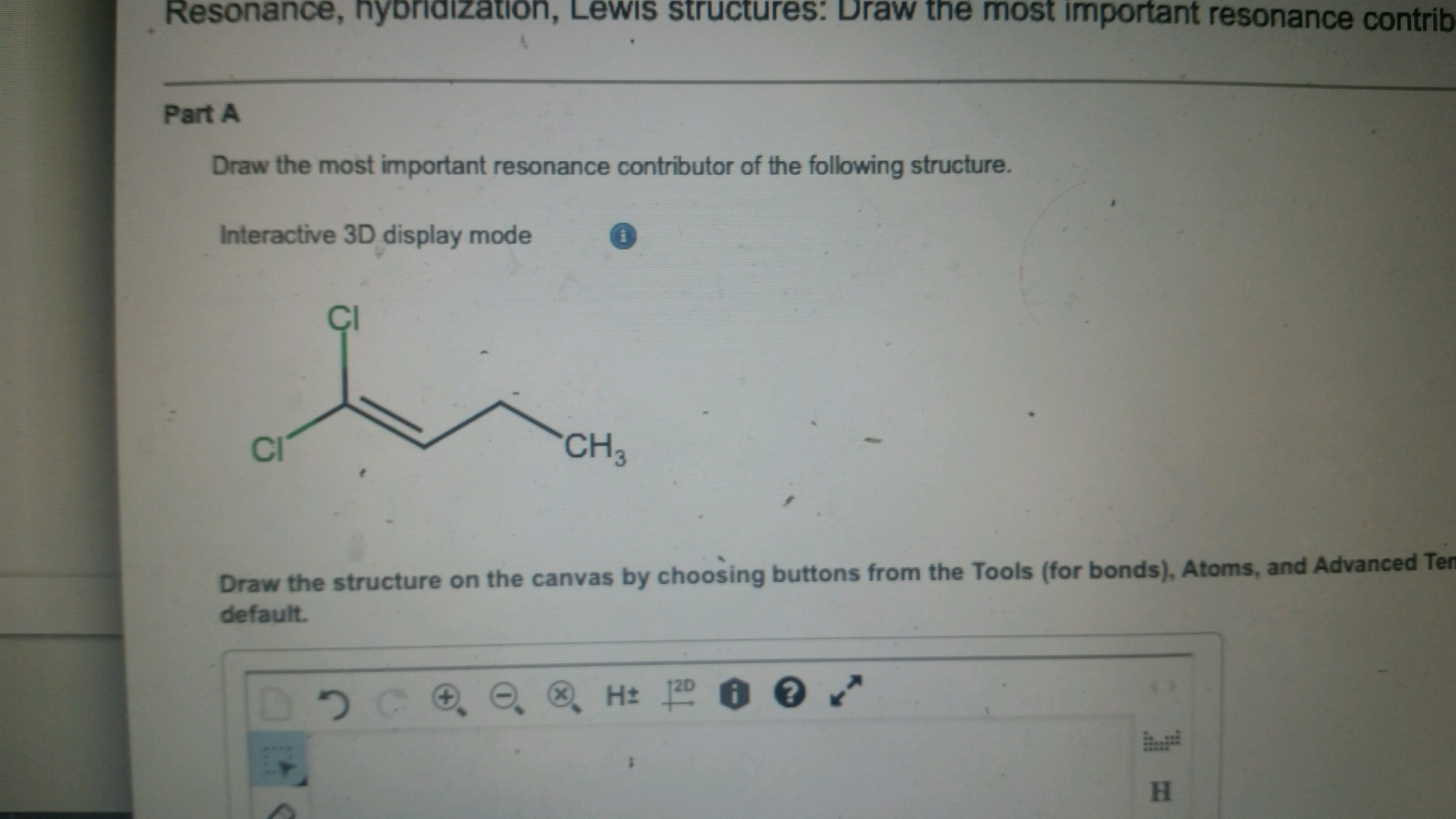
Solved Draw the most important resonance contributor of the
The Arrangement Of Atoms In A Molecule Or Ion Is Called Its.
Resonance Structures Are Various Forms Of.
This Is Important Because Neither Resonance Structure.
Resonance Contributors And The Resonance Hybrid.
Related Post: