Draw The Predominant Form Of Histidine At Ph 0
Draw The Predominant Form Of Histidine At Ph 0 - Draw the predominant form of each amino acid at ph 3.0. At low ph, such as ph=0, histidine exists primarily in its protonated form as h⁺. The ph most proteins would encounter). At ph = 6.02 there is equal amounts of h2a+ and ha. Draw the predominant form of histidine at the following ph values. Draw arginine in its predominant form at ph = 10.0. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. As the pka is very close to the ph, only small changes in the local environment can change the protonation state of the amino acid. Draw the structures of the major forms of histidine at ph values of 1, 4,7.6, and 11. This is close to physiological ph (i.e. Draw the predominant form of each amino acid at ph 3.0. Draw the predominant form of each amino acid at ph 7.0. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Draw histidine in its predominant form at. At ph = 6.02 there is equal amounts of h2a+ and ha. Web ph = 0 ph = 7 ph = 11 ho2. Here’s the best way to solve it. Draw the structure of the major form of phenylalanine at phvalues of 1, 5.5, and 11.(b) the isoelectric point of histidine is ph 7.6. This is close to physiological ph. Pka 3.86 nh2 o ho o oh glutamic acid pka 4.25 histidine nh2 o ho n+ n h h pka 6.04 nh2 o ho sh cysteine pka 8.35 lysine nh2 o ho nh3+ pka 10.79 nh2 o ho oh tyrosine pka 10.07 arginine nh2 o ho h n nh2 nh2+ pka 12.48. Draw the predominant form of each amino acid. Write the approximate pk_aof each group next to its position in your structure. Draw the predominate form of histidine at physiological ph. Draw the predominant form of each amino acid at ph 7.0. This problem has been solved! Draw each structure and analyze. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. At ph = 1.7 there is a equal amount of h3a2 + and h2a+. Draw the structure of the major form of phenylalanine at phvalues of 1, 5.5, and 11.(b) the isoelectric point of histidine is ph 7.6. Draw the predominate form of histidine at physiological. This is because the ph scale measures the concentration of hydrogen ions in a solution, and at low ph, there is an abundance of h⁺. (a) in ph 5 b, in ph 10 c, in ph 10.9 d, what is the net charge of histidine at the ph 10. Here’s the best way to solve it. Draw the structures of. Identify the amino acid with the lowest pl (try to solve this problem by inspecting their structures, rather than performing calculations). Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Draw each structure and analyze. This problem has been solved! At low ph, such as. The pl of histidine is $\boldsymbol {7.6}$. The pka of the side chain is 6.1. Web the conjugate acid (protonated form) of the imidazole side chain in histidine has a pk a of approximately 6.0. (a) in ph 5 b, in ph 10 c, in ph 10.9 d, what is the net charge of histidine at the ph 10. Video. Web (a) the isoelectric point (pi) of phenylalanine is ph 5.5. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Draw the structures of the major forms of histidine at ph values of 1, 4,7.6, and 11. Here’s the best way to solve it. Describe,. At ph 2 at ph 7.8 at ph 10 would you expect the amino acid tyrosine (a neutral amino acid) to be soluble at ph 8? At a ph ≈ (1.7+6.0)/2 = 3.9, 98 % is in the h2a+ form. Draw the predominate form of histidine at physiological ph. Web draw the predominant form of a given amino acid in. This occurs between pka2 and pka3, so we can calculate the pl as the average of these two values: The ph most proteins would encounter). Calculate the ratio of unprotonated to protonated histidine sidechains at ph 7.4. Draw each structure and analyze. The predominant form of histidine at ph=0 is h⁺. Web ph = 0 ph = 7 ph = 11 ho2. Web histidine is an essential amino acid that plays a vital role in the biosynthesis of proteins. (a) in ph 5 b, in ph 10 c, in ph 10.9 d, what is the net charge of histidine at the ph 10. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. The pka of the side chain is 6.1. Draw the predominate form of histidine at physiological ph. The imidazole ring of histidine is aromatic, which confers stability and makes it apolar at physiologic ph. The predominant form of histidine at ph=0 is: Video answers to similar questions. To find the pl (isoelectric point), we need to find the ph at which the net charge of histidine is 0. This problem has been solved!Solved 18. Draw the structure of histidine at the pH values
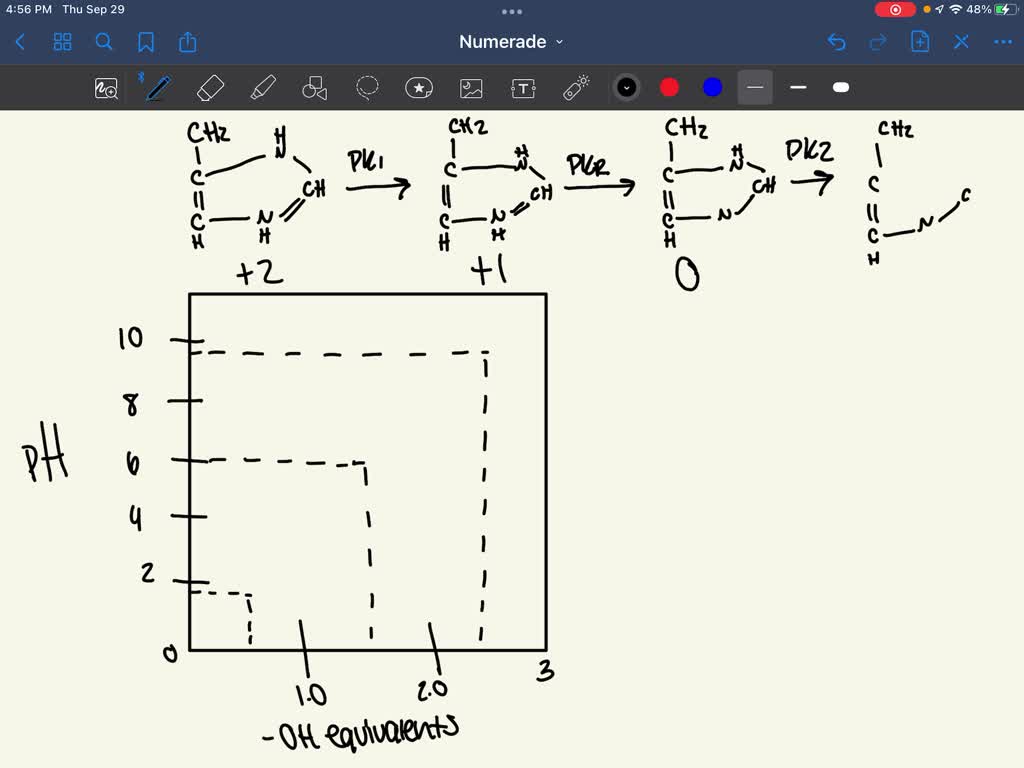
SOLVED a)Draw a Titration curve of histidine from pH 114. b) Draw
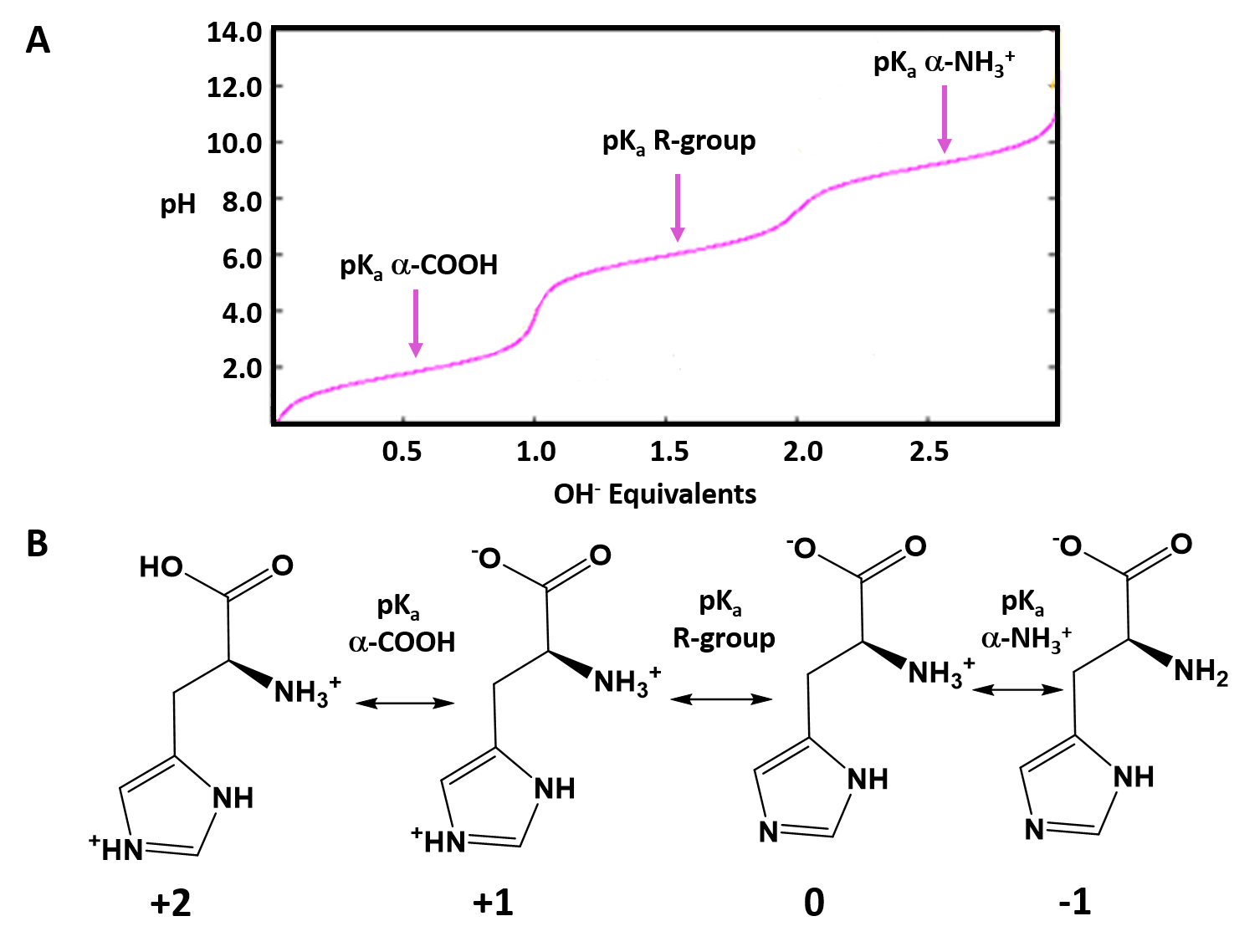
Histidine Titration Curve
[Solved] 1. Draw the structure of histidine that predominates at a pH
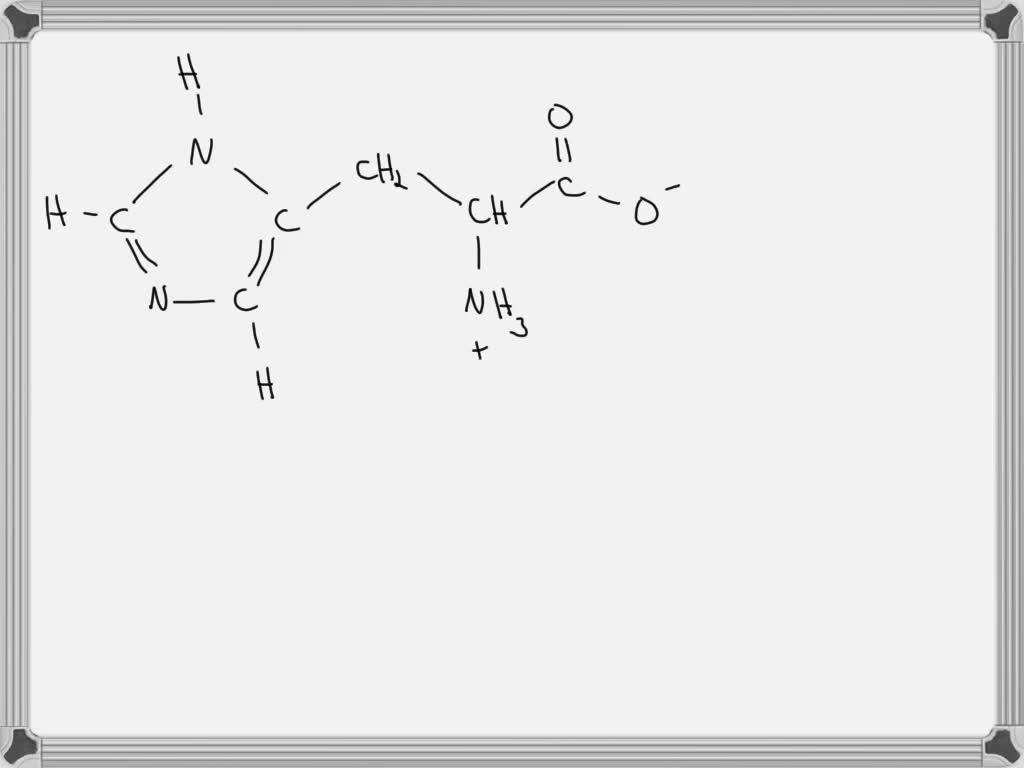
SOLVEDDraw the most predominant form of histidine at its isoelectric

Draw The Predominant Form Of Histidine At Ph 0 at Drawing
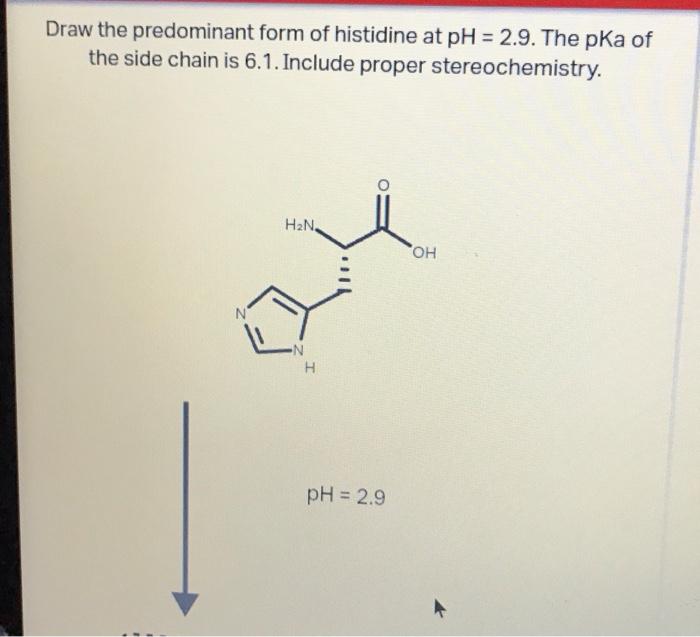
draw the predominant form of histidine at mathrmph29 the mathrmpka of
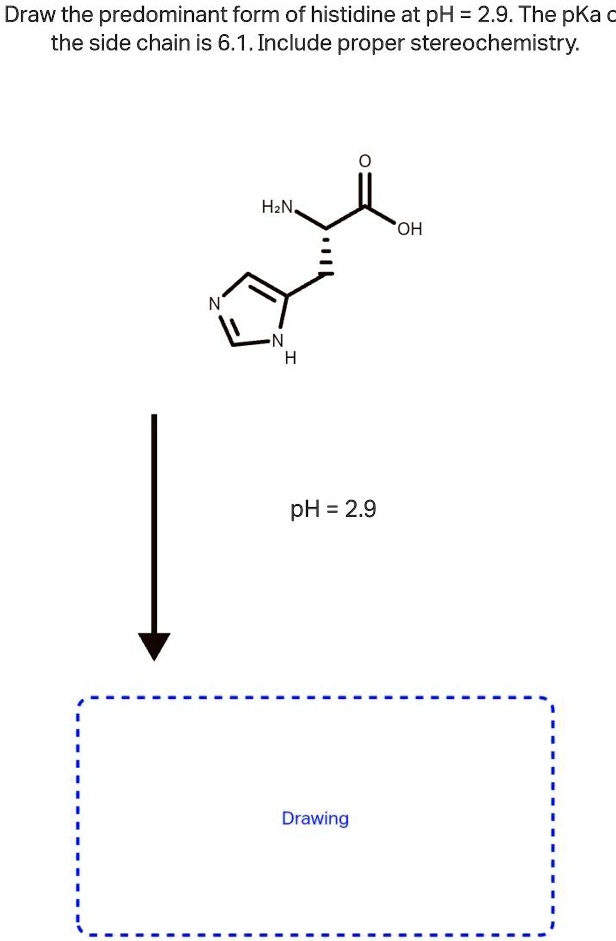
SOLVED Draw the predominant form of histidine at pH=2.9. The pKa of

Histidine chemical formula. Histidine chemical molecular structure
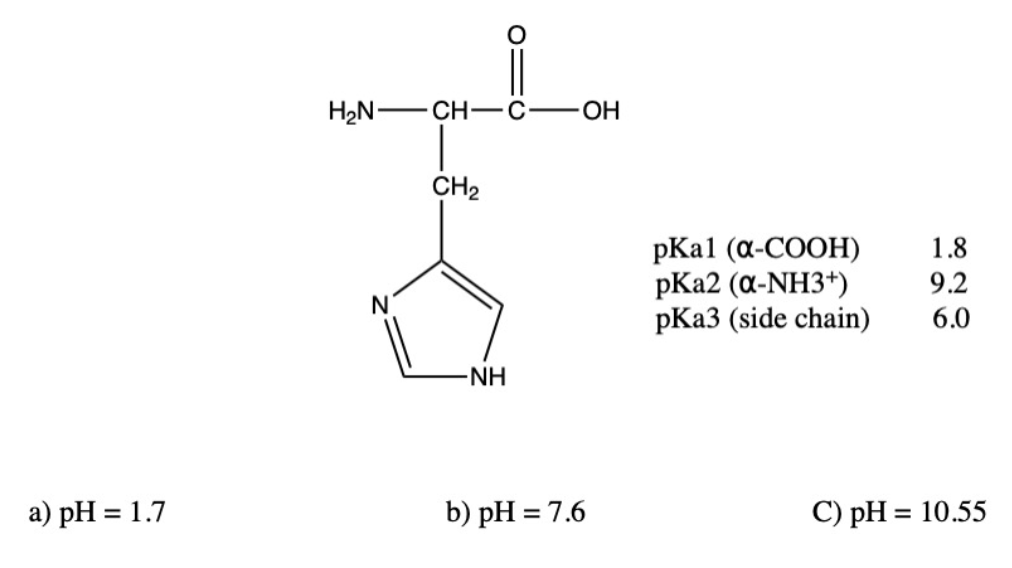
Solved Draw the structure of histidine at the following
Draw Histidine In Its Predominant Form At Ph = 7.0.
Draw The Predominant Form Of Histidine At Ph=0.
Below Ph = 1.7 (Pka1) H3A2+ Is The Most Abundant Specie.
At Ph = 1.7 There Is A Equal Amount Of H3A2 + And H2A+.
Related Post:
