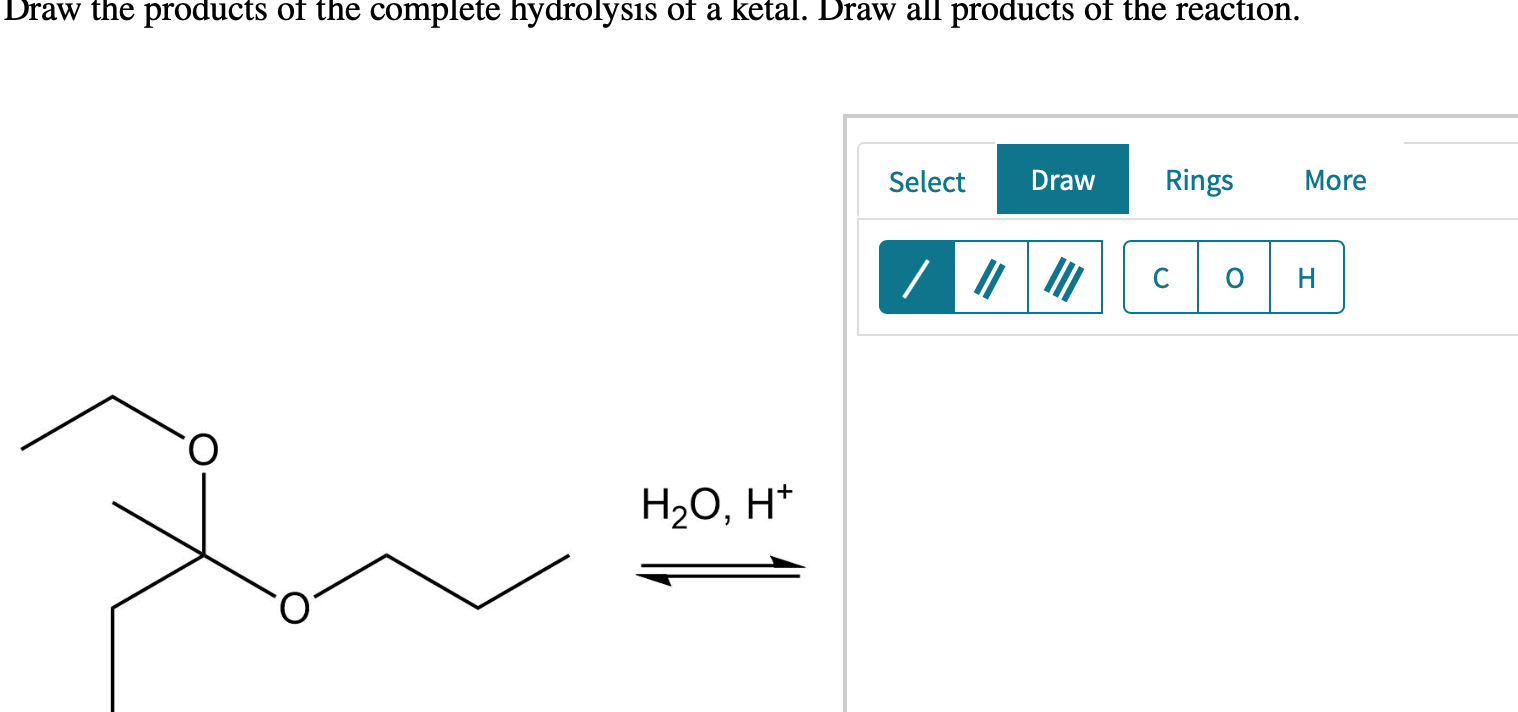
Draw The Products Of The Hydrolysis
Draw The Products Of The Hydrolysis - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web calculate the ph of a salt solution. The other reactants, and the products of hydrolysis, may be neutral molecules, as in most hydrolyses involving organic compounds, or ionic molecules, as in hydrolyses of salts, acids, and bases. Draw all products of the reaction. Hydrolysis reactions use water to breakdown polymers into monomers and is the opposite of dehydration synthesis, which forms water when synthesizing a polymer from monomers. A salt is formed between the reaction of an acid and a base. It is the reverse of dehydration synthesis or condensation reaction, where molecules combine to form larger molecules. Hydrolysis reactions break bonds and release energy. Web polysaccharides, such as starch, chitin, glycogen, and cellulose, can be broken down into monosaccharides. Identify the products of an acidic hydrolysis of an ester. Step 1/6identify the compound that will undergo hydrolysis.step 2/6determine the hydrolysis products by breaking the compound into its constituent ions or molecules.step 3/6write the hydrolysis products. It is the reverse of dehydration synthesis or condensation reaction, where molecules combine to form larger molecules. Draw the structures of the products from the hydrolysis of each of the following with naoh. Draw. Condensation and hydrolysis reactions are chemical reactions involving organic compounds. This occurs through the process of hydrolysis, which uses water to break the bonds between monosaccharides. Draw all products of the reaction. This problem has been solved! Draw the products of the hydrolysis shown. Draw the products of the hydrolysis shown. Web calculate the ph of a salt solution. Identify the products of a basic hydrolysis of an ester. Hydrolysis reactions break bonds and release energy. Hydrolysis reactions use water to breakdown polymers into monomers and is the opposite of dehydration synthesis, which forms water when synthesizing a polymer from monomers. There are quite a few steps in this reaction but fortunately, you don’t need to remember all the steps in order to predict the structure of the aldehyde and ketone of an acetal hydrolysis. Identify the products of an acidic hydrolysis of an ester. Draw the products of the complete hydrolysis of a ketal. The entire molecule changes its structure. The other reactants, and the products of hydrolysis, may be neutral molecules, as in most hydrolyses involving organic compounds, or ionic molecules, as in hydrolyses of salts, acids, and bases. The entire molecule changes its structure as new bonds are formed. Draw the products of the hydrolysis shown. Draw all products of the reaction. Label the identity of each one. Draw the products of the complete hydrolysis of an acetal. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Label the identity of each one of the products (glycerol or give the name of the fatty acid). Dive into the detailed mechanism of this vital biological reaction. Web this problem has been solved! Web distinguish between condensation and hydrolysis reactions. Web explore the fascinating process of atp hydrolysis! Condensation and hydrolysis reactions are chemical reactions involving organic compounds. Hydrolysis reactions break bonds and release energy. The entire molecule changes its structure as new bonds are formed. Write a paragraph or two discussing what happened at the molecular level during this experiment, and focusing on the the analysis of your soap. Web draw all the hydrolysis products of each of the following. Web hydrolysis under acidic conditions requires strong acids such as sulfuric or hydrochloric, and temperatures of about \(100^\text{o}\) for several hours. Draw the products of. Want to join the conversation? Draw all products of the reaction. Web hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Identify the products of an acidic hydrolysis of an ester. Select draw rings more erase cop. Here’s the best way to solve it. Identify the products of an acidic hydrolysis of an ester. Describe the typical reaction that takes place with esters. There are 3 steps to solve this one. Want to join the conversation? Generate products of the hydrolysis of amides. Discover how water interacts with atp molecules, triggering a release of energy as bonds break and electrons find a more comfortable state. Esters are neutral compounds, unlike. A salt is formed between the reaction of an acid and a base. There are 3 steps to solve this one. This problem has been solved! The reaction can be represented as: Esters are neutral compounds, unlike the acids from which they are formed. This occurs through the process of hydrolysis, which uses water to break the bonds between monosaccharides. H + + oh − ⇌ h 2o. Identify the products of an acidic hydrolysis of an ester. Draw the products of the complete hydrolysis of an acetal. Draw the line angle structure of propanal, draw the structure or write the formula of the reagent above the arrow and draw. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Incorrect draw the product of the reaction. Web hydrolysis under acidic conditions requires strong acids such as sulfuric or hydrochloric, and temperatures of about \(100^\text{o}\) for several hours.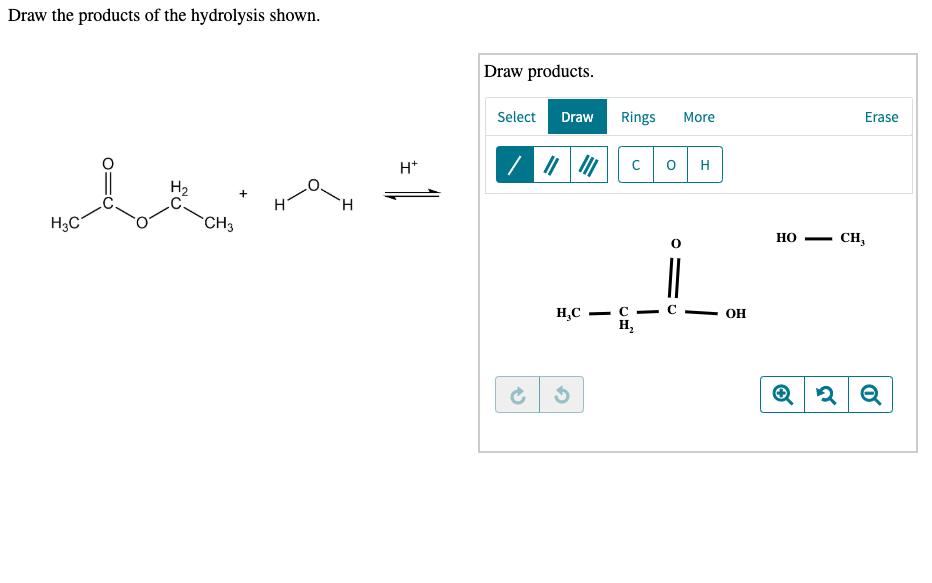
Solved Draw the products of the hydrolysis shown. Draw
Draw The Products Formed From The Ester Hydrolysis Reaction Shown

Hydrolysis Reaction Definition, Equation, and Applications
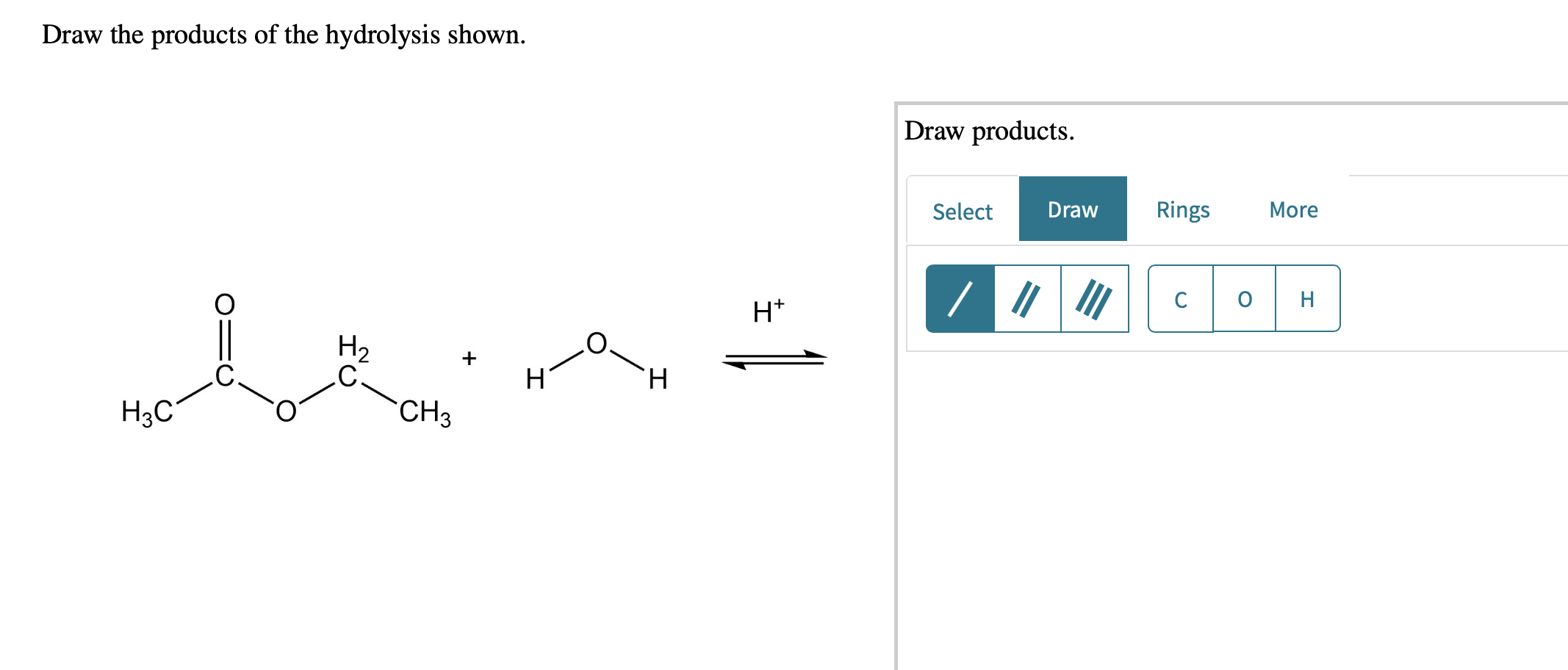
Solved Draw the products of the hydrolysis shown. Draw
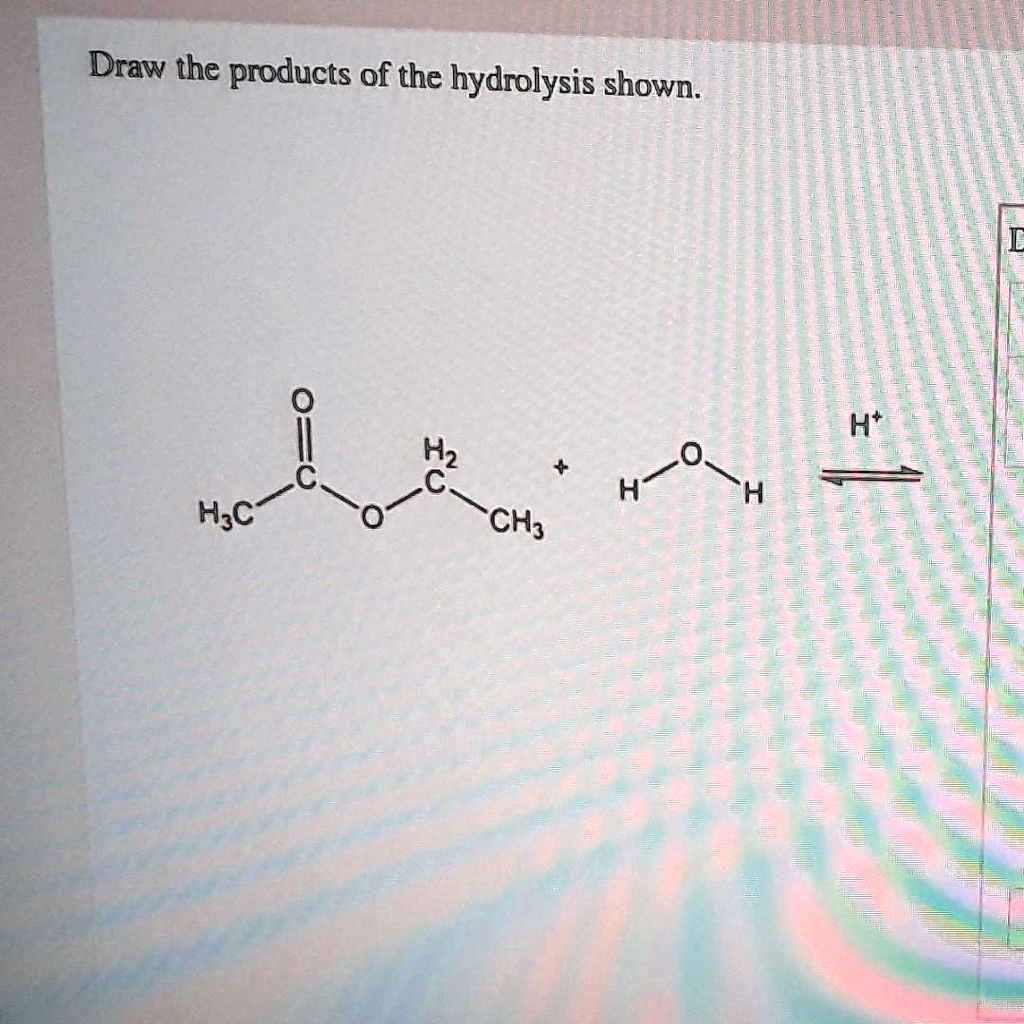
Draw the products of the hydrolysis shownIHtH2HFH;c … SolvedLib
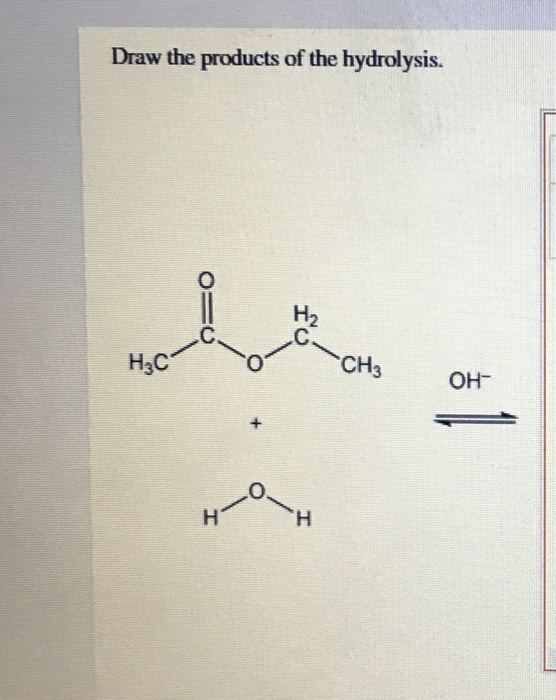
Solved Draw the products of the hydrolysis. НАС CH₃ OH
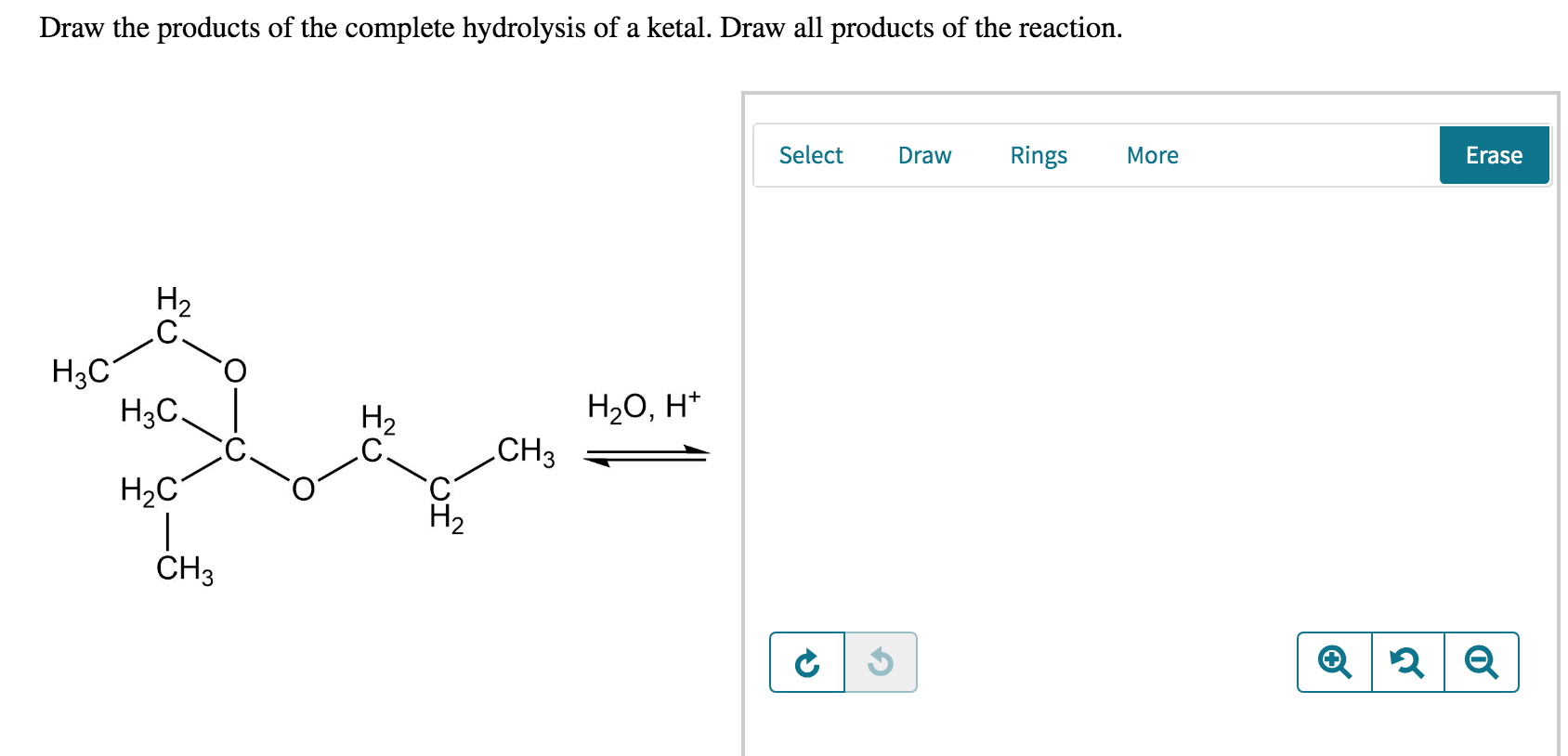
Draw The Products Of Each Hydrolysis Reaction The Expert

Hydrolysis Definition, Examples, & Facts Britannica
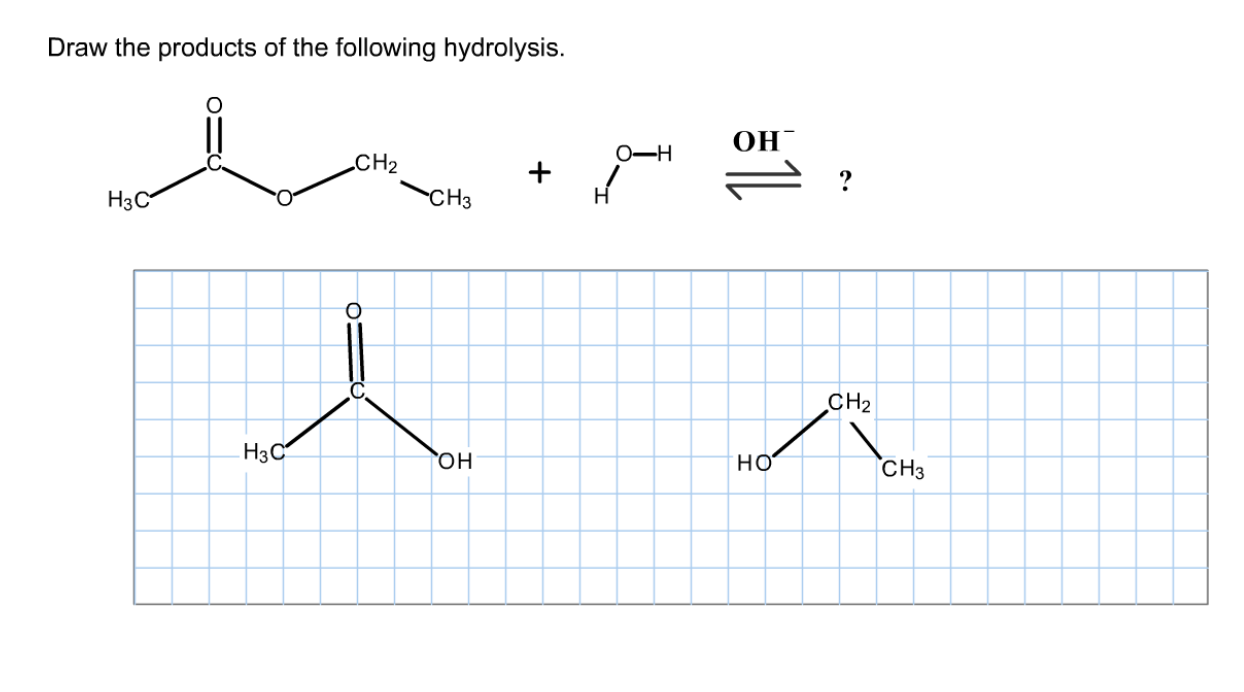
Solved Draw The Products Of The Following Hydrolysis
Solved Draw the products of the complete hydrolysis of a
Calculate The Concentrations Of Various Ions In A Salt Solution.
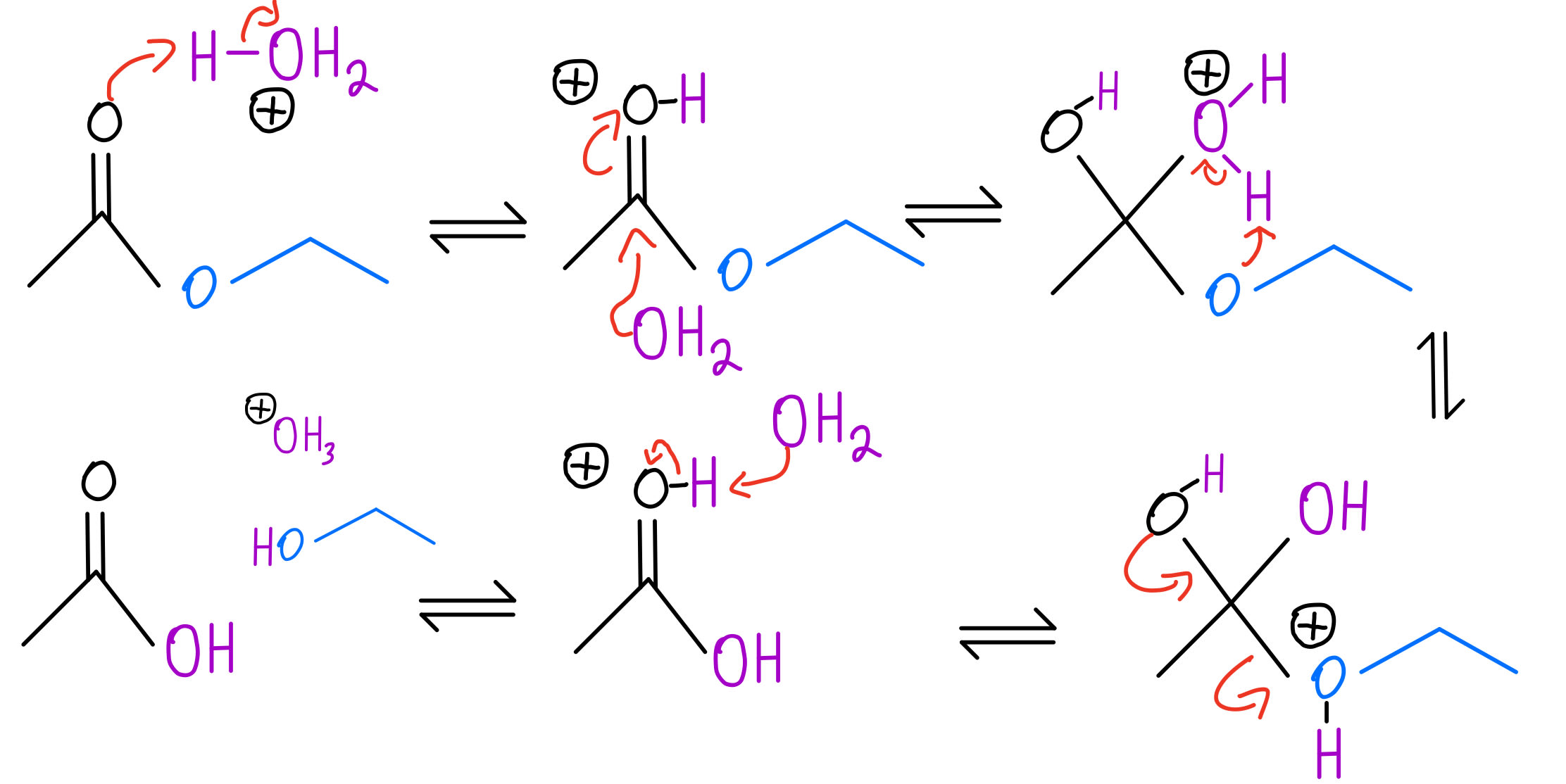
The Mechanism Involves Protonation Of The Amide On Oxygen Followed By Attack Of Water On The Carbonyl Carbon.
Identify The Products Of A Basic Hydrolysis Of An Ester.
Want To Join The Conversation?
Related Post: