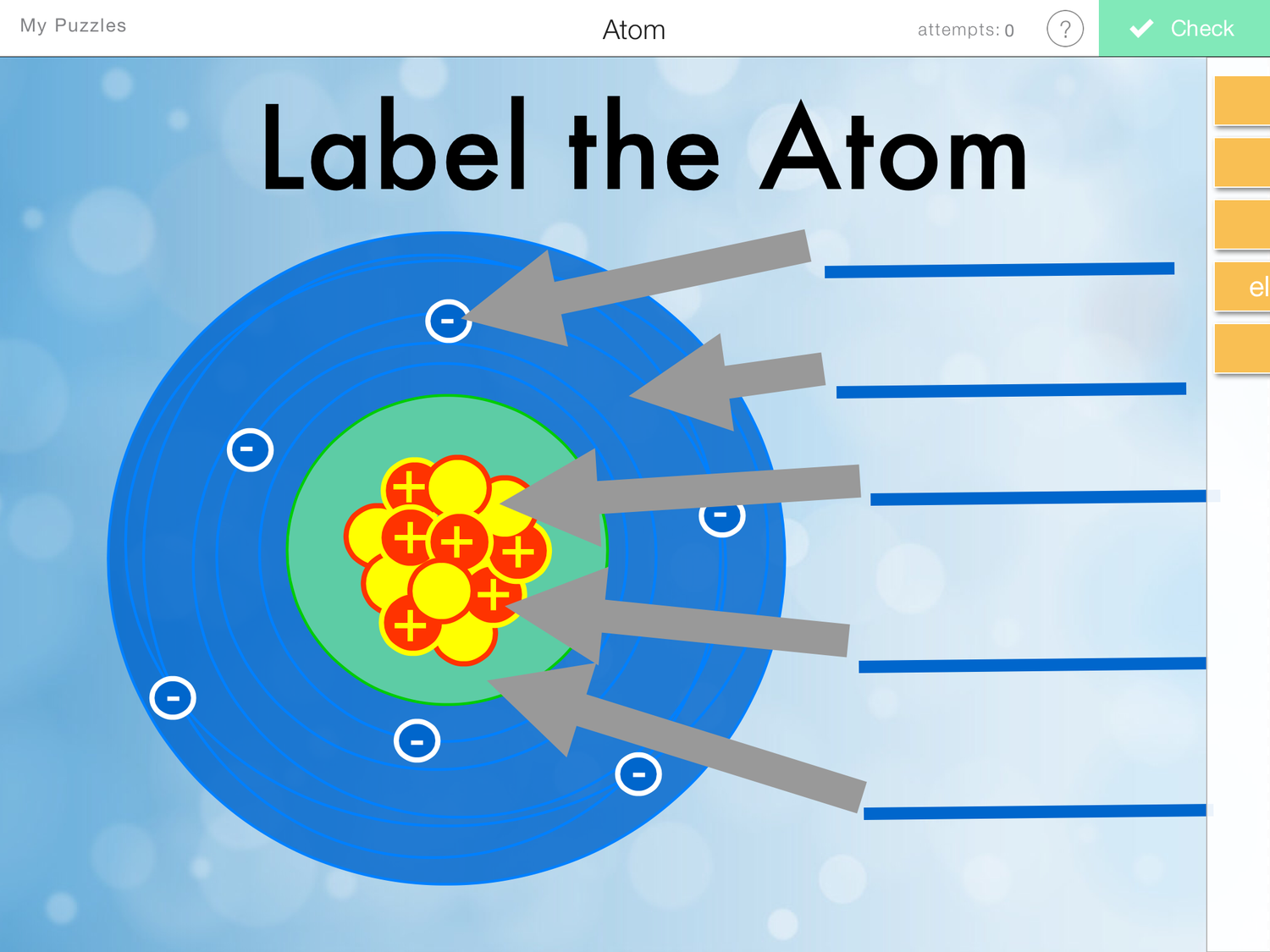
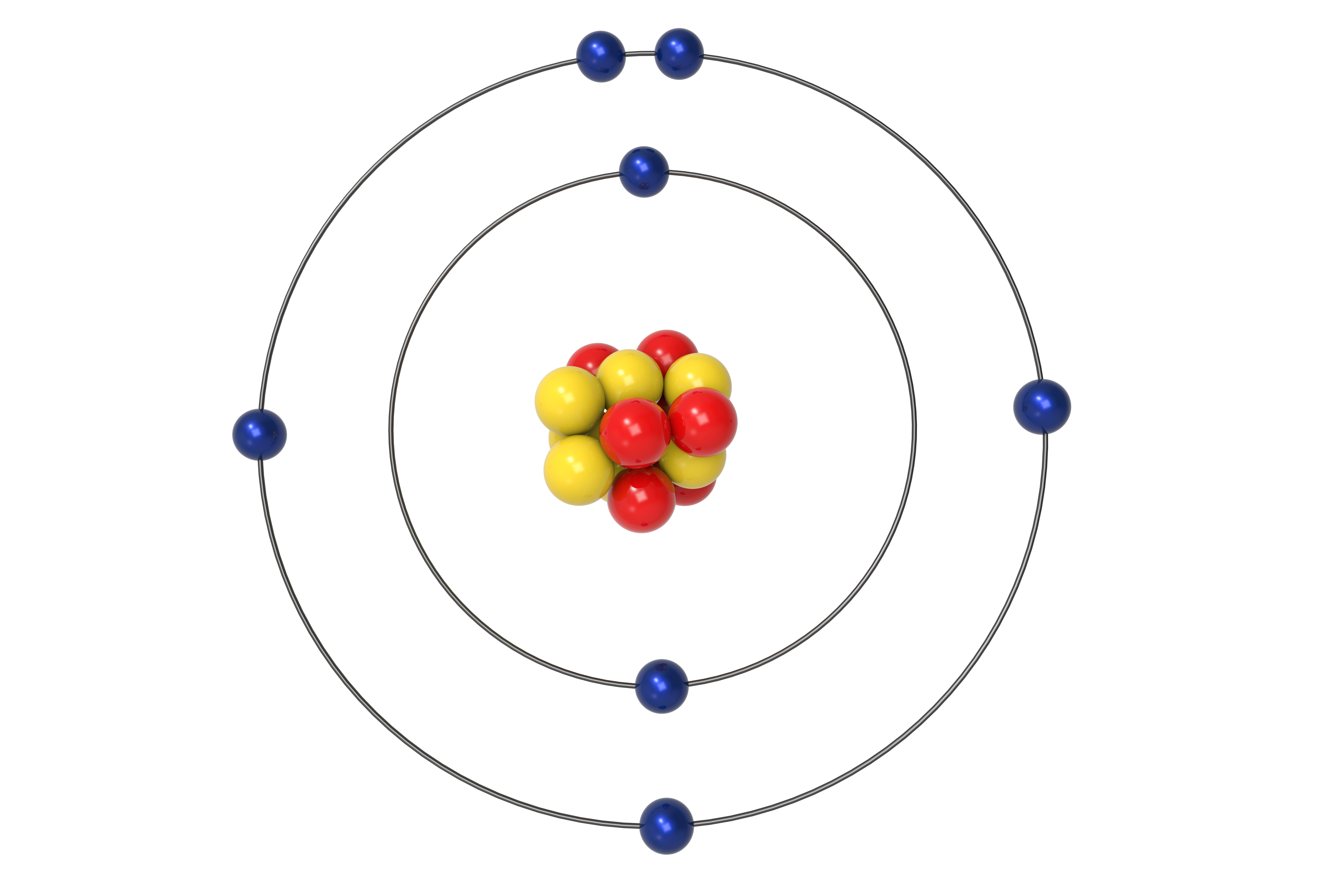
Draw The Structure Of An Atom
Draw The Structure Of An Atom - (choice a) protons, neutrons, and photons. The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. What three particles make up an atom? Naming and drawing amines is shared under a not declared license. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Web the three main parts of an atom are protons, neutrons, and electrons. Web if you want (or need) to draw a model of an atom, we'll show you how!#atom #atomicstructure #atomicmodel #howtodraw Science > biology library > chemistry of life > elements and atoms. See all videos for this article. Mass is measured in kilograms (kg) or grams (g). Democritus came up with the concept that matter is composed of atoms. All living organisms and nonliving objects found on earth are made of trillions and trillions of atoms. The electrons are negatively charged particles that orbit the nucleus’s centre. The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and. Atomic structure and electrons made easy. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Since the iodine is added as a 1− anion, the number of. Amines are classified as primary, secondary, or tertiary by the number. Carbon (atomic number 6) has six electrons. Web the number of electrons in an atom is always the same as the number of protons, so atoms are electrically. The history of atomic chemistry (opens a modal) discovery of the electron and nucleus (opens a modal) atomic models. Some helocs offer a discounted teaser rate for a period before switching to. The atom is the basic building block of matter. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). In many cases, it's wise to avoid these and opt for. Atoms are the smallest particle of matter than cannot be further subdivided using chemical. Web we recommend using the latest version of chrome, firefox, safari, or edge. John dalton, in 1800 proposed the first scientific. Try rotating the device so that it is in a landscape position. Some helocs offer a discounted teaser rate for a period before switching to a higher fully indexed rate later on. Atoms can lose or gain electrons. Atoms can combine with other atoms to form molecules but cannot be divided into smaller parts by ordinary chemical processes. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The electrons are negatively charged particles that orbit the nucleus’s centre. Atomic number, atomic mass, and isotopes. Rutherford’s gold foil experiment (opens. Rutherford’s gold foil experiment (opens a modal) atomic number, mass number, and isotopes Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. When. When one says an atom is electrically neutral, it means that. Since the iodine is added as a 1− anion, the number of. John dalton, in 1800 proposed the first scientific. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web we recommend you. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Web atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral). Then play a game to test your ideas! When drawing orbital diagrams,. Following hydrogen is the noble gas helium, which has an atomic number of 2. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.. Web if you want (or need) to draw a model of an atom, we'll show you how!#atom #atomicstructure #atomicmodel #howtodraw In its structure below, highlight the atom. The protons and neutrons are located in the center of the atom, while the. The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Web atom, the basic building block of all matter and chemistry. Web the electron configuration and the orbital diagram are: Mass is measured in kilograms (kg) or grams (g). To indicate they are protons, draw them as circles with plus signs contained inside. Web we recommend using the latest version of chrome, firefox, safari, or edge. What three particles make up an atom? Atoms can lose or gain electrons. Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. Same skeleton (including h) same skeleton (excluding h) Protons, neutrons, and photons (choice b) positrons, neutrons, and electrons. Following hydrogen is the noble gas helium, which has an atomic number of 2. Explore an atom's interior to discover the layout of its nucleus, protons, and electrons.
How to Draw an Atom Really Easy Drawing Tutorial

Atom Definition, Structure & Parts with Labeled Diagram
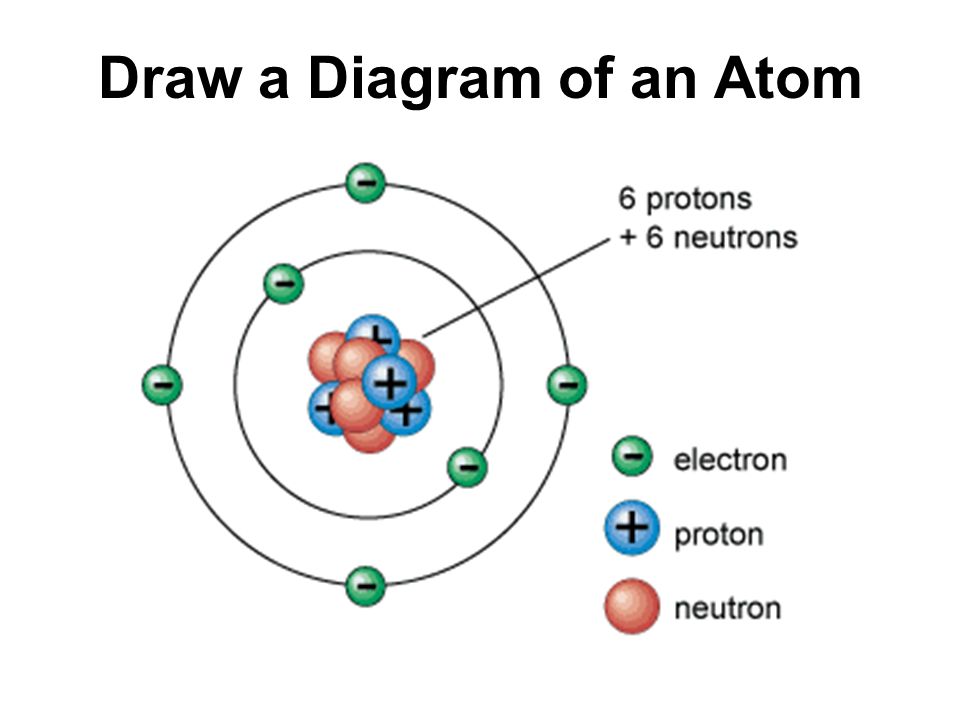
Skills Practice AMAZING 8TH GRADE SCIENTISTS
Parts Of An Atom Worksheet

How to draw Atom structure diagram step by step l Atomic structure

Learn the Parts of an Atom
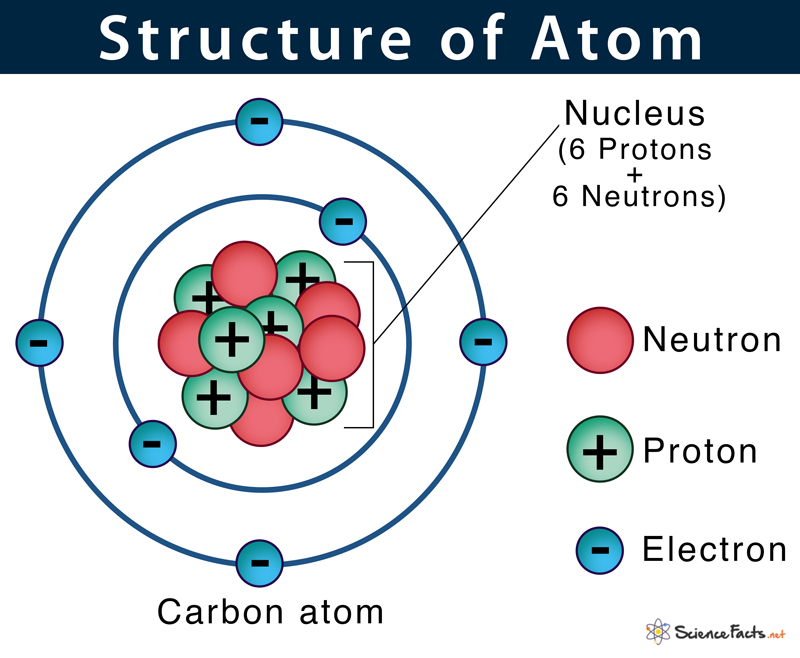
Atom Definition, Structure & Parts with Labeled Diagram
Label The Parts Of A Atom
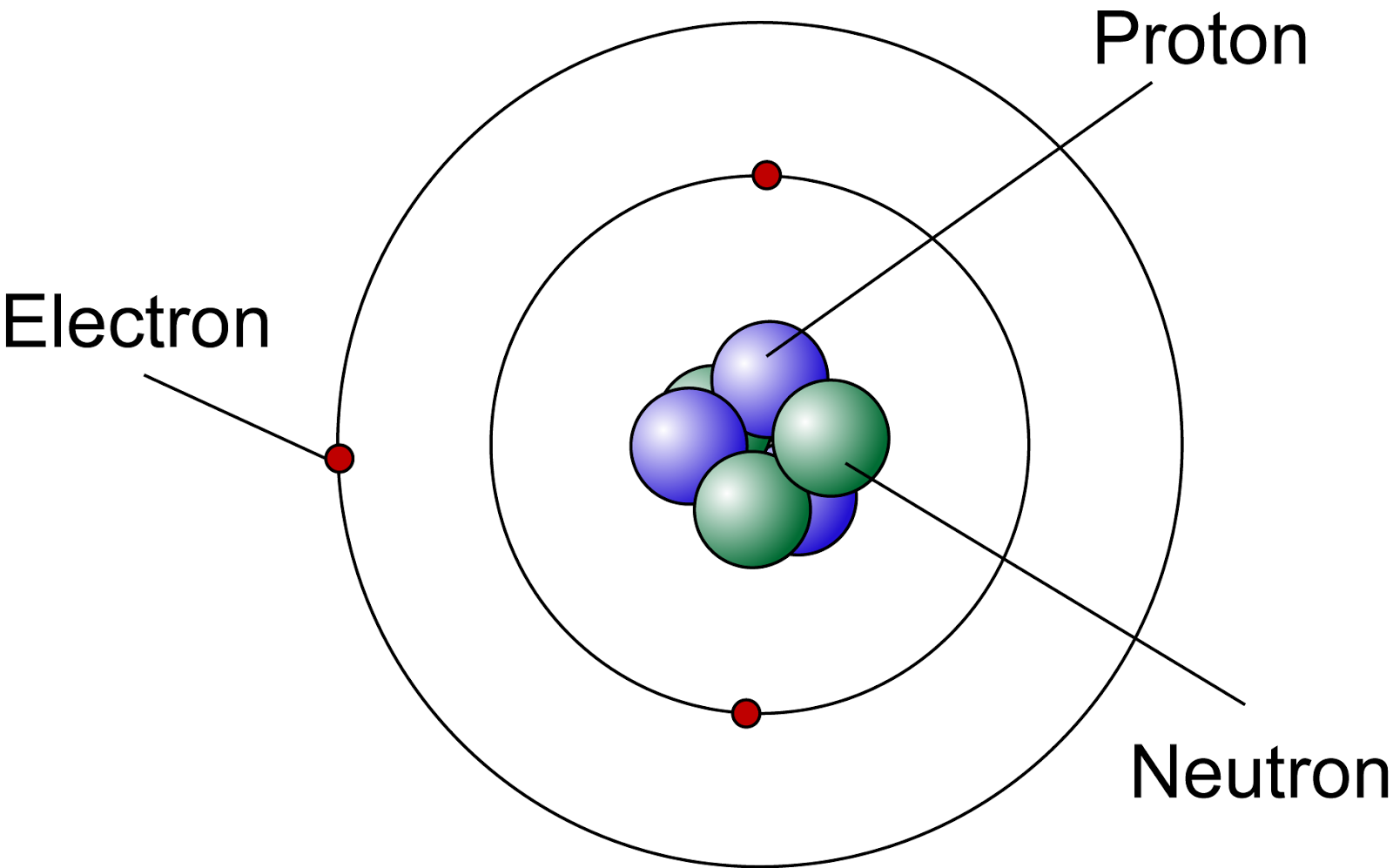
Lets Get Inside An Atom!! The Science Station
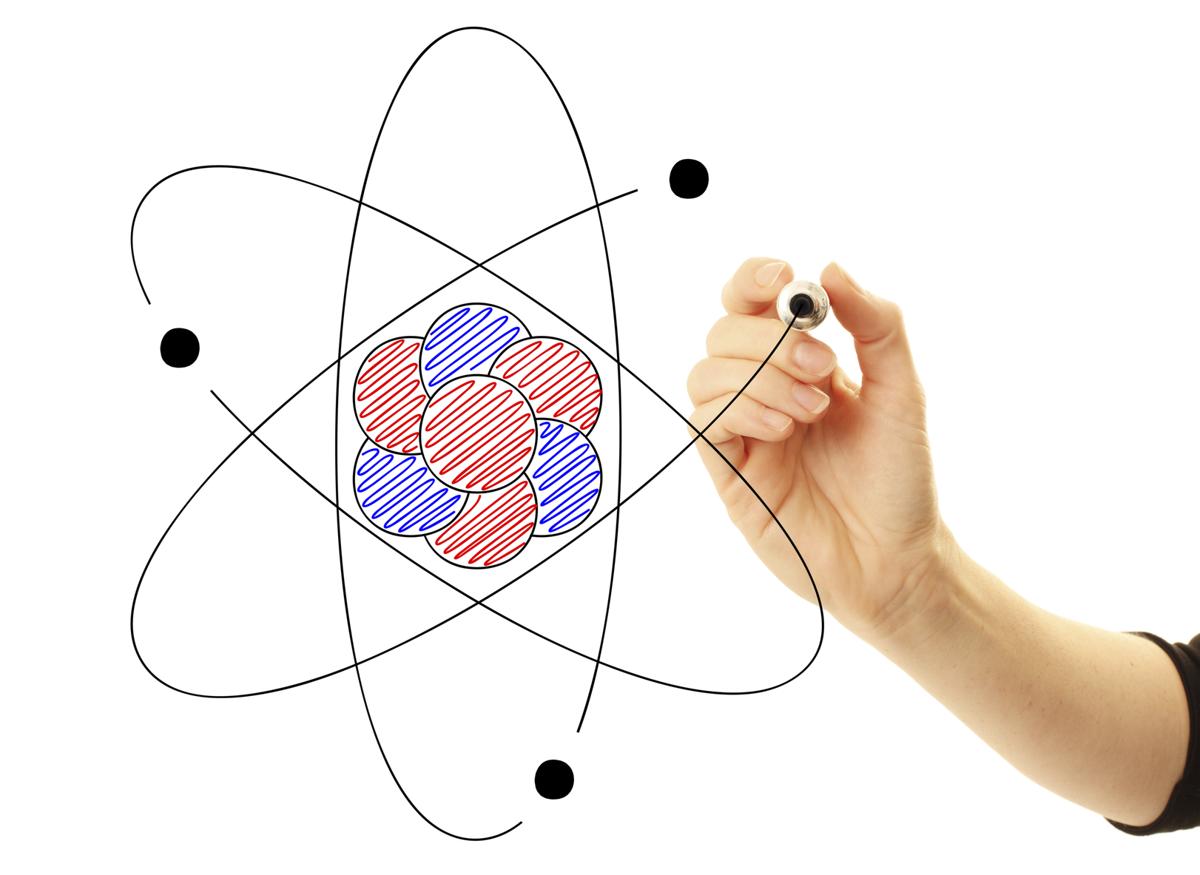
The Structure of an Atom Explained With a Labeled Diagram Science Struck
Naming And Drawing Amines Is Shared Under A Not Declared License.
The Center Of An Atom Is The Nucleus And One Or More Electrons Surrounding The Nucleus.
Most Of An Atom Is Just Empty Space And Consists Of A Positively Charged Nucleus Of Protons And Neutrons Surrounded By A Cloud Of Negatively Charged Electrons.
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
Related Post: