Draw The Structure Of Butane
Draw The Structure Of Butane - Same skeleton (including h) same skeleton (excluding h) Online lectures from kahn academy. Let’s see how to do it. Figure shows the structure of isobutane. The molecular formula of butane can be expanded to c h 3 c h 2 c h 2 c h 3. Follow some steps for drawing the lewis dot structure for c4h10. (do not show the hydrogen atoms.) a) draw the complete structure of butane. Its molecule is composed of hydrogens and carbons entirely. Count total valence electron in c4h10 As you know, isomers are molecules that have the same molecular. An expanded formula, a condensed formula,. Its molecule is composed of hydrogens and carbons entirely. Follow some steps for drawing the lewis dot structure for c4h10. This means that two methylpropane structure formulas are identical to two isobutane structure. Let’s see how to do it. Figure shows the structure of isobutane. Count total valence electron in c4h10 And if we stare down the carbon two three bond, so here i'm rotating the molecule so we stare down the carbon two three bond. Structure of n butane or neo butane. The butane molecule contains a total of 13 bond (s). They indicate only the connections among atoms. The strain energy of the gauche interaction is about 0.9 kcal/mol. Its molecule is composed of hydrogens and carbons entirely. The drawing of the butane (c4h10) lewis structure is an easy and simple process. An expanded formula, a condensed formula,. The drawing of the butane (c4h10) lewis structure is an easy and simple process. Online lectures from kahn academy. If no reaction occurs, draw the organic starting material. It is also used as a feedstock for ethylene and butadiene production. Web draw the structure (s) of the major neutral organic product (s) obtained after workup of the following reaction. Web draw the structure (s) of the major neutral organic product (s) obtained after workup of the following reaction. Butane is a saturated hydrocarbon containing 4 carbons, with an unbranched structure. And have the following lewis structures: • draw one structure per sketcher. Online lectures from kahn academy. Same skeleton (including h) same skeleton (excluding h) If no reaction occurs, draw the organic starting material. Butane is a saturated hydrocarbon containing 4 carbons, with an unbranched structure. The butane molecule contains a total of 13 bond (s). (do not show the hydrogen atoms.) select draw rings more erase select draw rings more erase с h с h 2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. • draw one structure per sketcher. Web draw the structure (s) of the major neutral organic product (s) obtained after workup of the following reaction. Web butane can be split into two isomers. Web butane is an organic compound with the formula c4h10. Follow some steps for drawing the lewis dot structure for c4h10. Butane may be stored in pits in the earth capped by metal domes and in underground chambers. Figure shows the structure of isobutane. If no reaction occurs, draw the organic starting material. Figure 20.3 the same structure can be represented three different ways: A) draw the complete structure of butane. Web a chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. This is a staggered confirmation of butane. Online lectures from kahn academy. Web we recommend you use a larger device to draw your structure. A) draw the complete structure of butane. Web figure 20.3 shows three different ways to draw the same structure. Br₂ h₂o you do not have to consider stereochemistry. And have the following lewis structures: Figure 20.3 the same structure can be represented three different ways: If no reaction occurs, draw the organic starting material. The strain energy of the gauche interaction is about 0.9 kcal/mol. Web now we add enough hydrogen atoms to give each carbon four bonds: Try rotating the device so that it is in a landscape position. The butane molecule contains a total of 13 bond (s). Count total valence electron in c4h10 Images of the chemical structure of butane are given below: Butane is primarily used as a gasoline mixture, either alone or in a propane mixture. Web this problem has been solved! Web we recommend you use a larger device to draw your structure. And if we stare down the carbon two three bond, so here i'm rotating the molecule so we stare down the carbon two three bond. Structure of n butane or neo butane. The molecular formula of butane can be expanded to c h 3 c h 2 c h 2 c h 3. Web figure 20.3 shows three different ways to draw the same structure. Figure 20.3 the same structure can be represented three different ways: Follow some steps for drawing the lewis dot structure for c4h10.
Butane Molecular Geometry Hybridization Molecular Weight

Draw electron dot structure of butane.
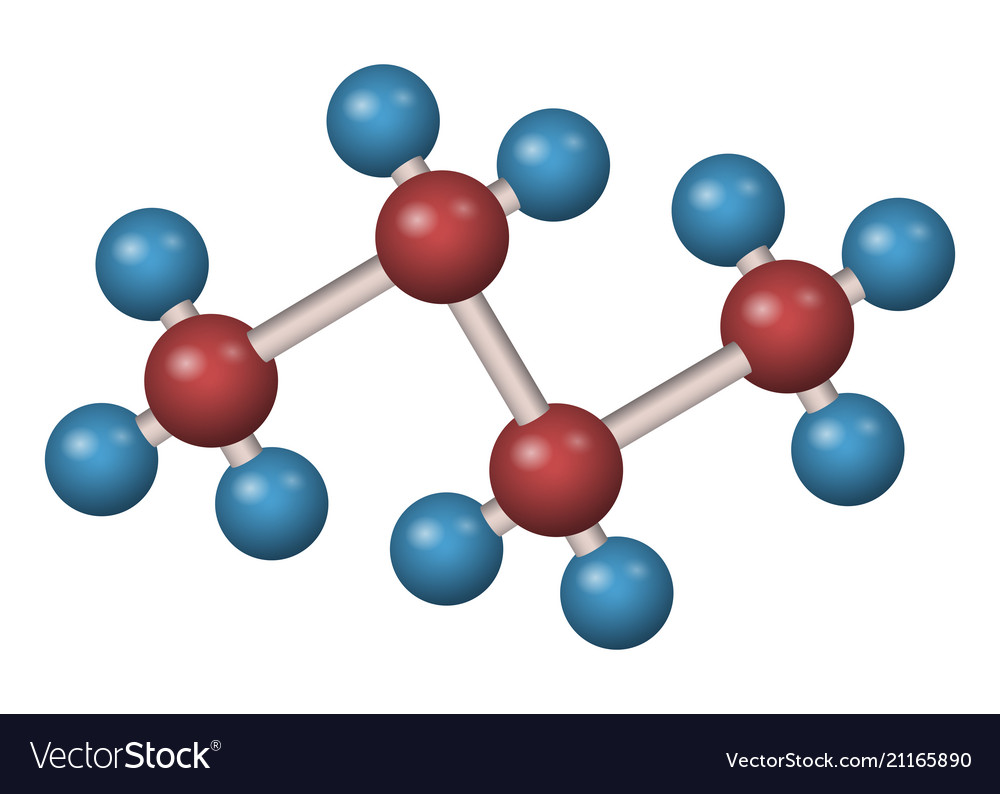
Butane molecule is a 3d formula Royalty Free Vector Image
[Solved] Draw butane (C 4 H 10 ). What class of organic molecule is

Molecular model of Butane Stock Photo 52583963 Alamy

Butane stock illustration. Illustration of education 83615830
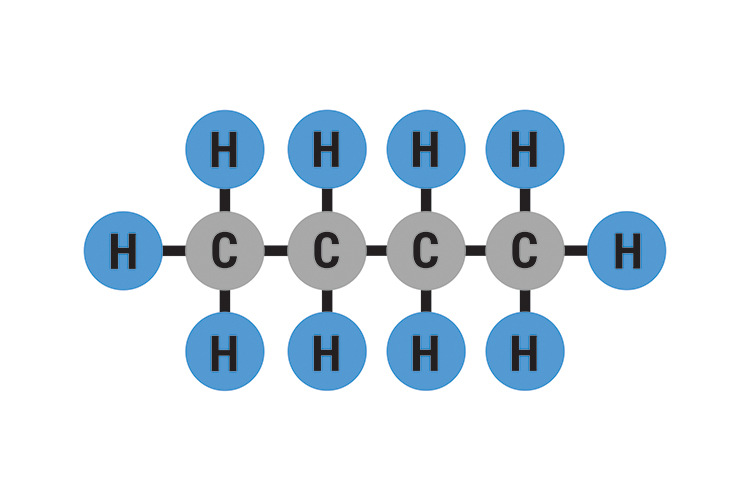
Shapes of Molecules Chemistry Coach
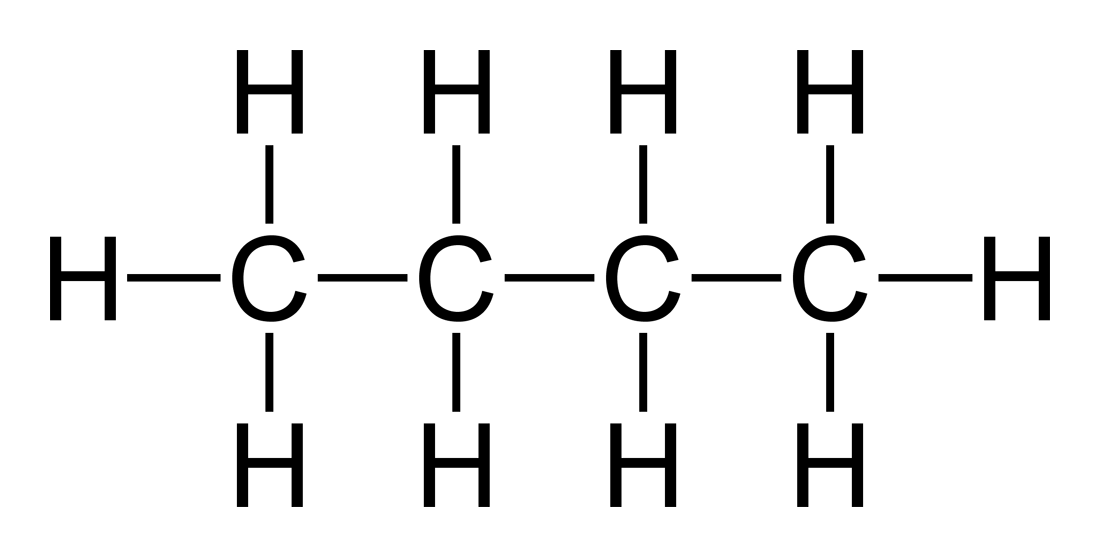
The molecular structure of Butane and formula structure
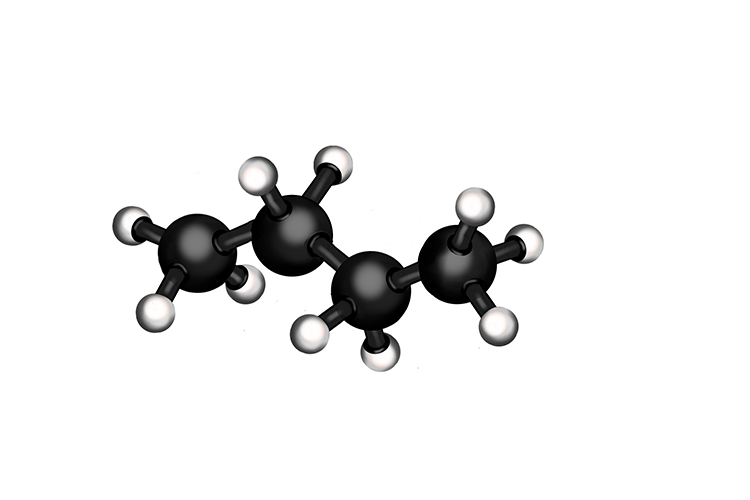
The molecular structure of Butane and formula structure

The illustration of the butane structural formula Stock Vector Image
There Are 3 Steps To Solve This One.
Butane Is A Saturated Hydrocarbon Containing 4 Carbons, With An Unbranched Structure.
Web Butane Is An Organic Compound With The Formula C4H10.
Same Skeleton (Including H) Same Skeleton (Excluding H)
Related Post: