Drawing Hybrid Orbitals
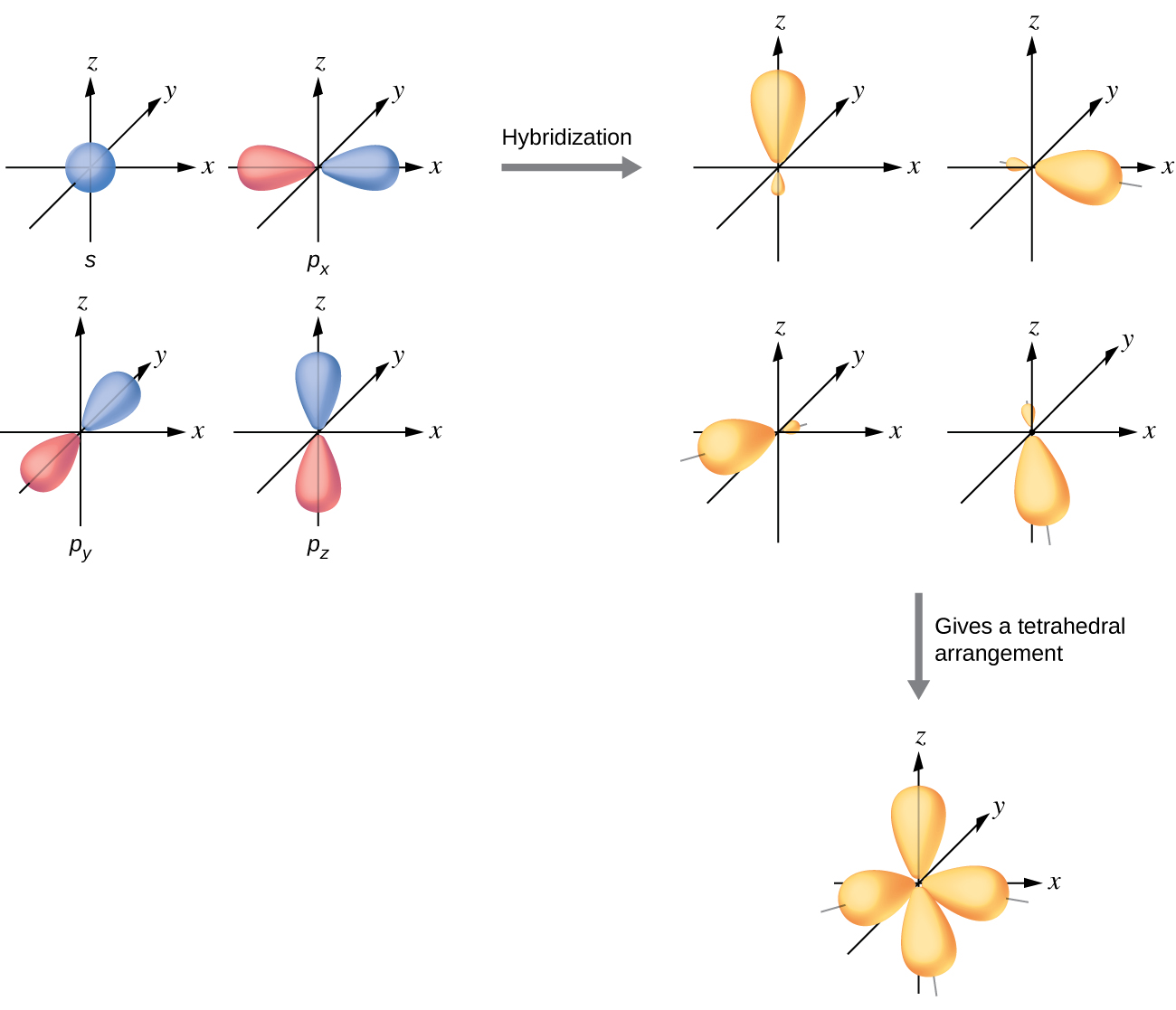
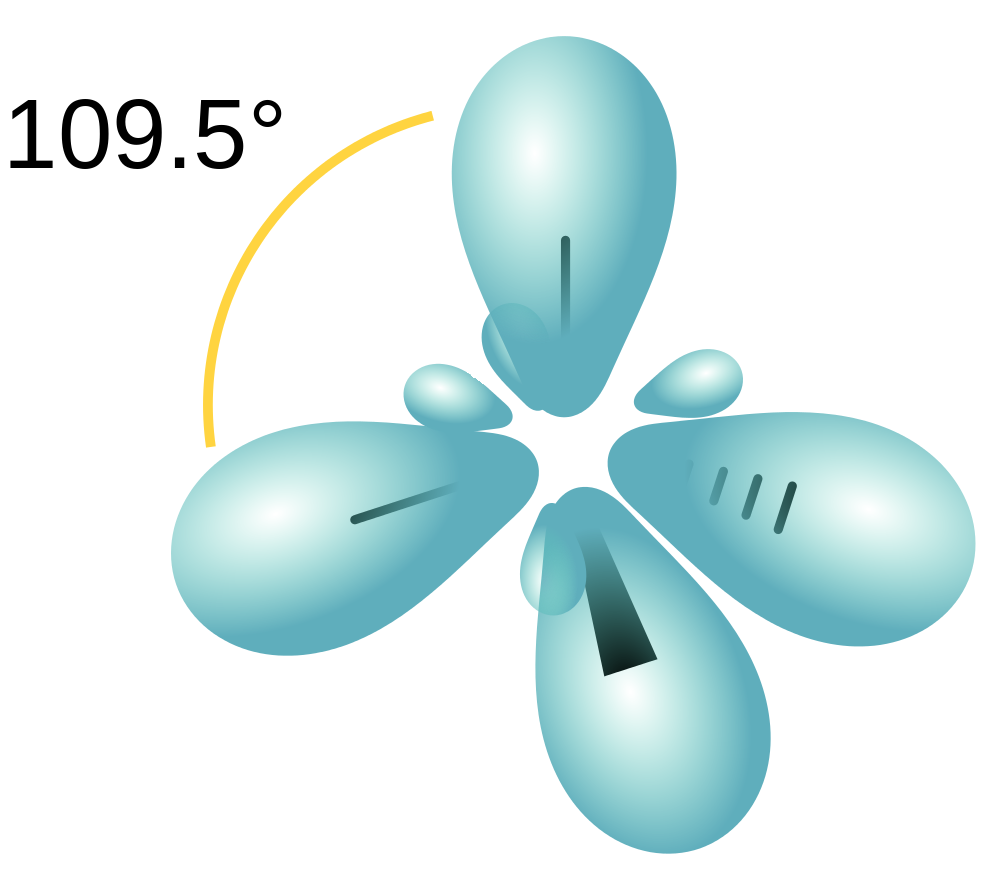
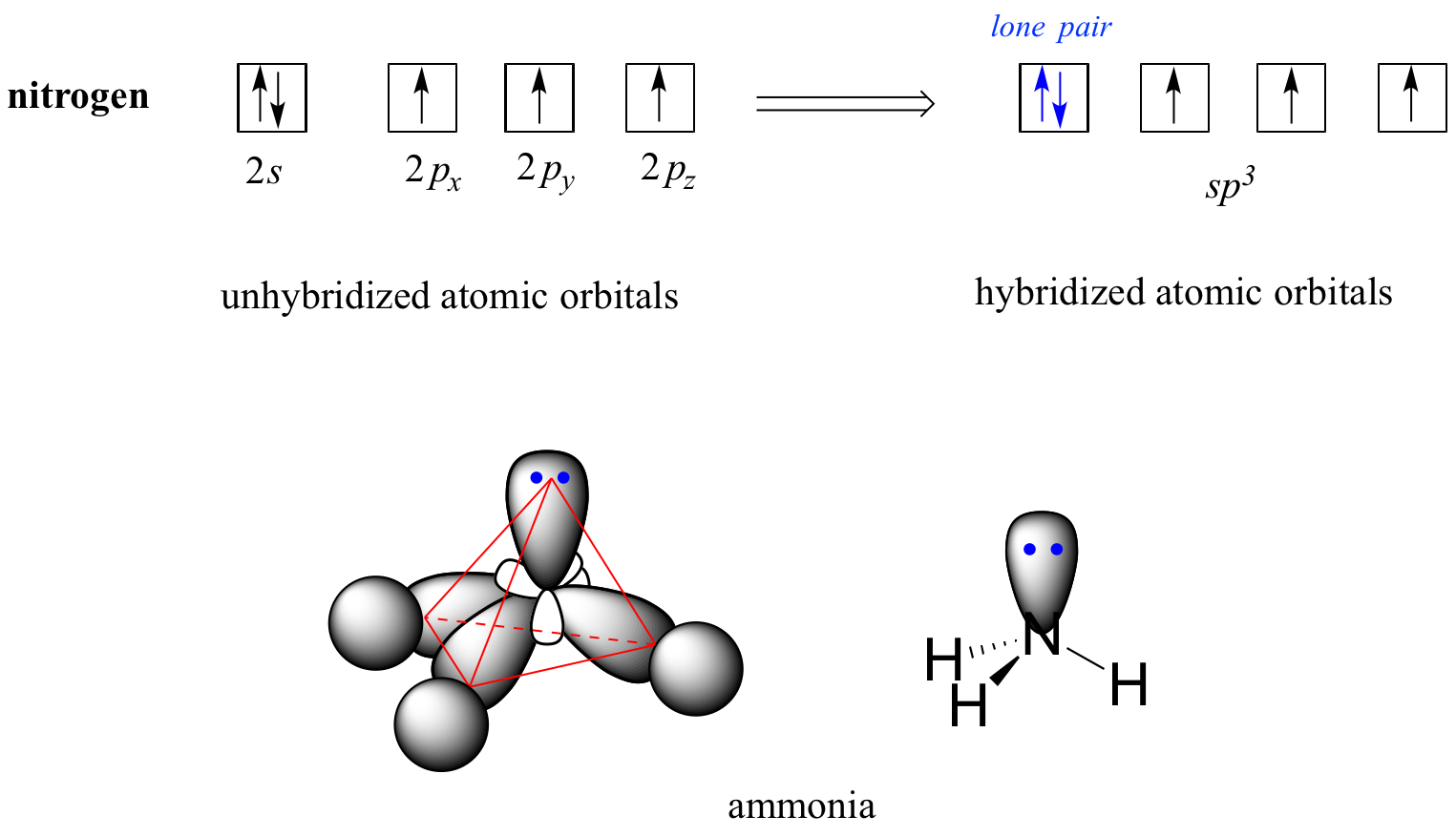
Drawing Hybrid Orbitals - Directory of chem help asap videos:. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. Draw a figure showing the bonding picture for the imine below. Carbon dioxide, co 2 , has a linear shape. In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Web using the ns orbital, all three np orbitals, and one (n − 1)d orbital gives a set of five sp 3 d hybrid orbitals (the five hybrid orbitals that result when one s three p and one d orbitals are combined (hybridized)). This organic chemistry video tutorial explains the hybridization of atomic orbitals. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. A set of hybrid orbitals is generated by combining atomic orbitals. Web the formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination of the orbitals to give a new set of (usually) equivalent orbitals that are oriented properly to form bonds. Draw a figure showing the bonding picture for the imine below. Hybrid orbitals overlap to form σ bonds. Web steps to draw hybrid orbital diagrams for a molecule. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. Web the formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the combination of the orbitals to give a. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Learn about sp² hybridized orbitals and pi bonds. Recall the valence electron configuration of a carbon atom: Web a set of hybrid orbitals is generated by combining atomic orbitals. It discusses how to determine the. The three sp 2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120°between them. Why does the other p electron stay by itself? Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Unhybridized orbitals overlap to form π bonds. It. Web hybridization of an s orbital with all three p orbitals (p x, p y, and p z) results in four sp 3 hybrid orbitals. What is the hybridization around the central carbon atom in co 2 ? Web hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Now let’s look more carefully at. Web steps to draw hybrid orbital diagrams for a molecule. Determine the hybrid orbitals associated with various molecular geometries. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Sp3 hybrid orbitals and tetrahedral bonding. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set. Web the formation of hybrid atomic orbitals can be viewed as occurring via promotion of an electron from a filled ns 2 subshell to an empty np or (n − 1)d valence orbital, followed by hybridization, the. Read through the provided information, and sketch the lewis dot diagram of the provided compound. Web explain the concept of atomic orbital hybridization. 1.9m views 3 years ago new ap & general chemistry video playlist. Show how hybrid orbitals are involved in the molecules methane, water, and ammonia. What is the hybridization around the central carbon atom in co 2. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set. This organic chemistry video tutorial explains the hybridization of atomic orbitals. Directory of chem help asap videos:. What is the hybridization around the central carbon atom in co 2 ? Sp3 hybrid orbitals and tetrahedral bonding. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. Learn about sp² hybridized orbitals and pi bonds. Now let’s look more carefully at bonding in organic molecules, starting with methane, ch. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Web steps to draw hybrid orbital diagrams for a molecule. Carbon dioxide, co 2 , has a linear shape. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. This 109.5 o arrangement gives tetrahedral geometry (figure 4). A set of hybrid orbitals is generated by combining atomic orbitals. Want to join the conversation? Web explain the concept of atomic orbital hybridization. Determine the hybrid orbitals associated with various molecular geometries. Now let’s look more carefully at bonding in organic molecules, starting with methane, ch 4. The carbon atoms of c2h2 are sp hybridized. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Hybrid orbitals overlap to form σ bonds. Web using the ns orbital, all three np orbitals, and one (n − 1)d orbital gives a set of five sp 3 d hybrid orbitals (the five hybrid orbitals that result when one s three p and one d orbitals are combined (hybridized)).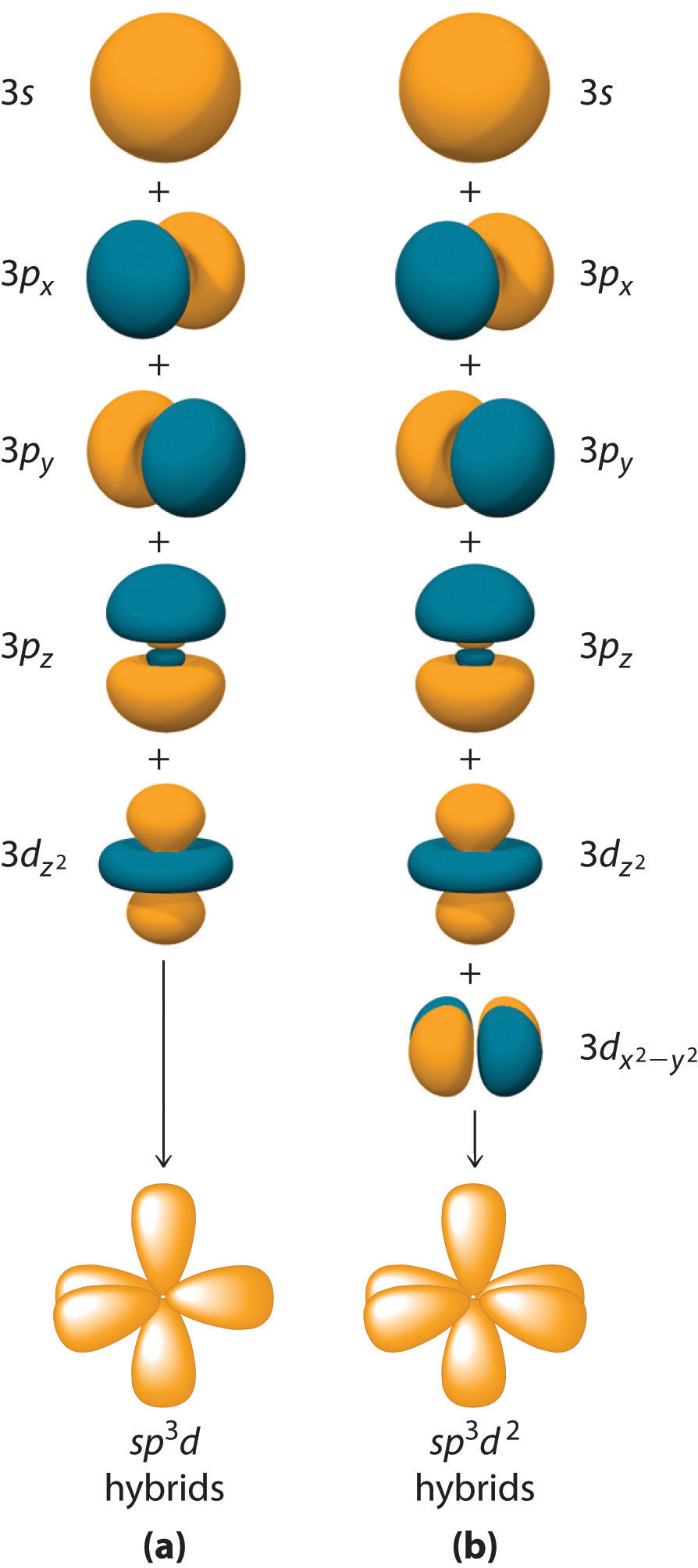
9.5 Hybrid Orbitals Chemistry LibreTexts
8.2 Hybrid Atomic Orbitals Chemistry

Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp, Sp2, Sp3
Valence Bond Theory and Hybrid Orbitals Introductory Chemistry
2.2 Hybrid orbitals Chemistry LibreTexts
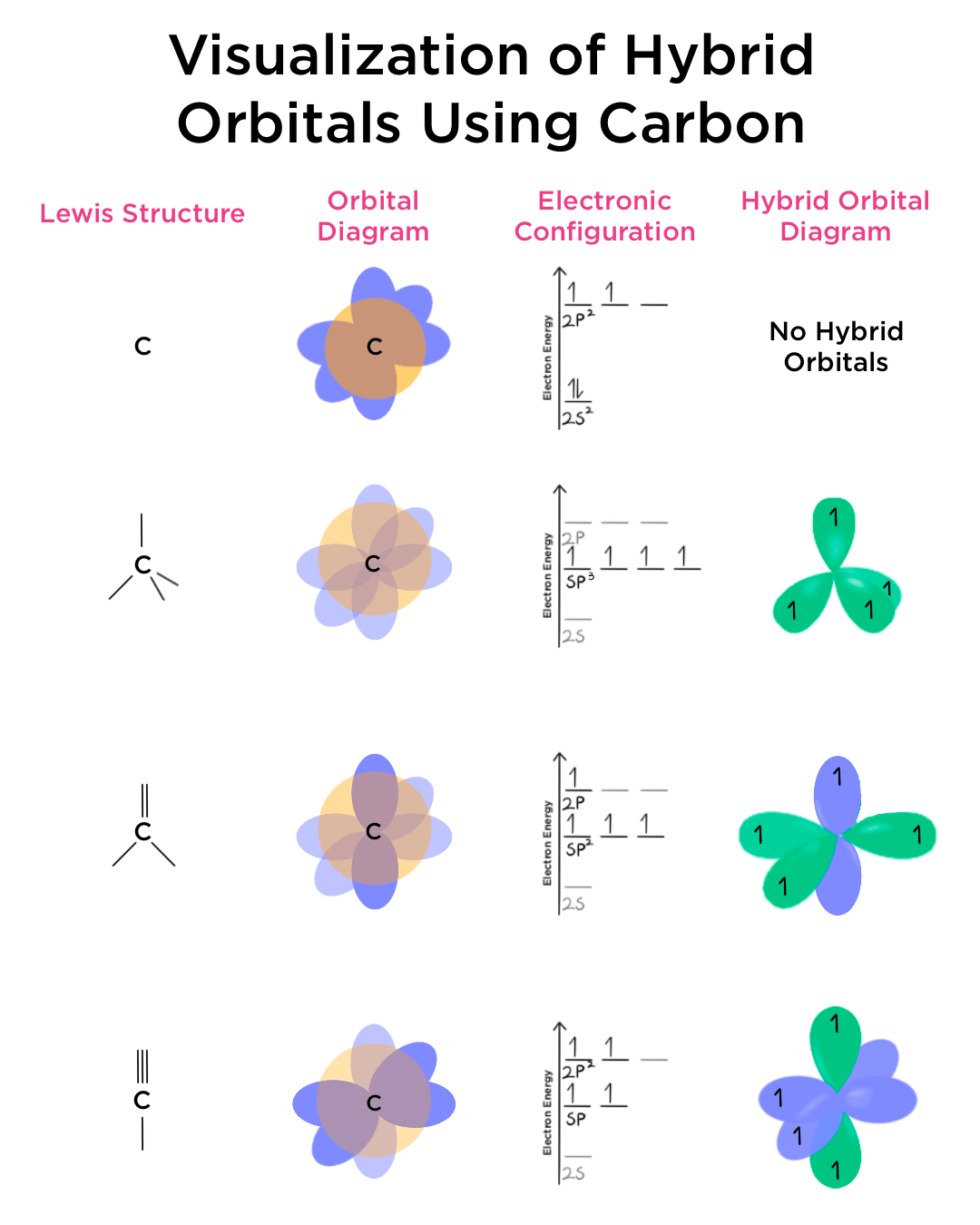
Hybrid Orbitals — Overview & Examples Expii
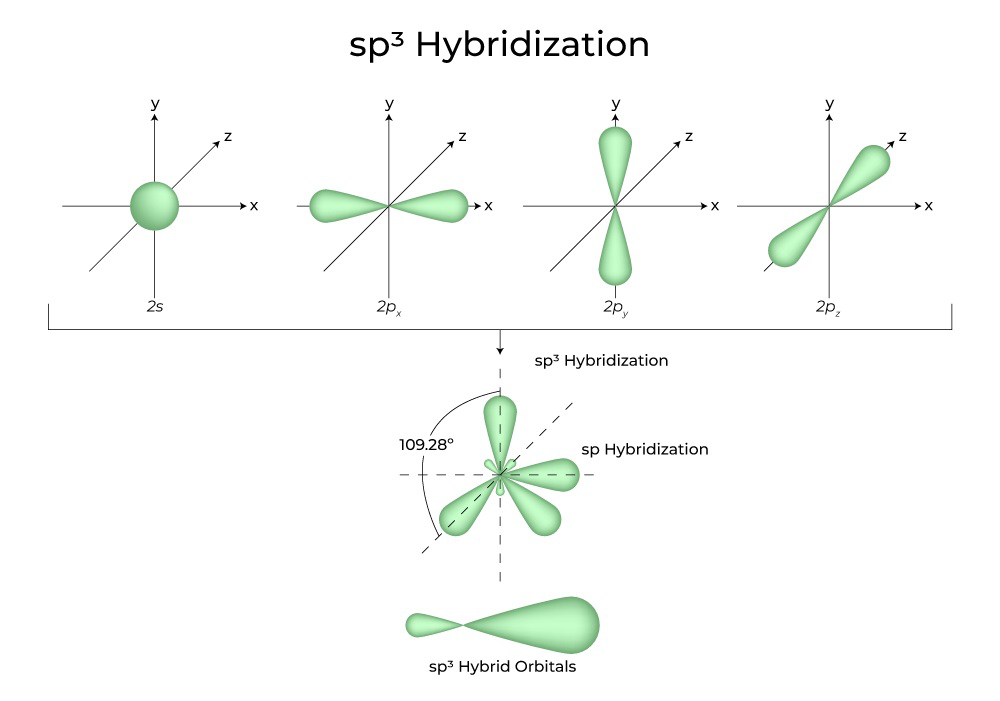
Hybridization Definition, Types, Rules, Examples

1 Formation And Geometry Of Hybrid Orbitals Sp3, Sp2, Sp Images, Stock
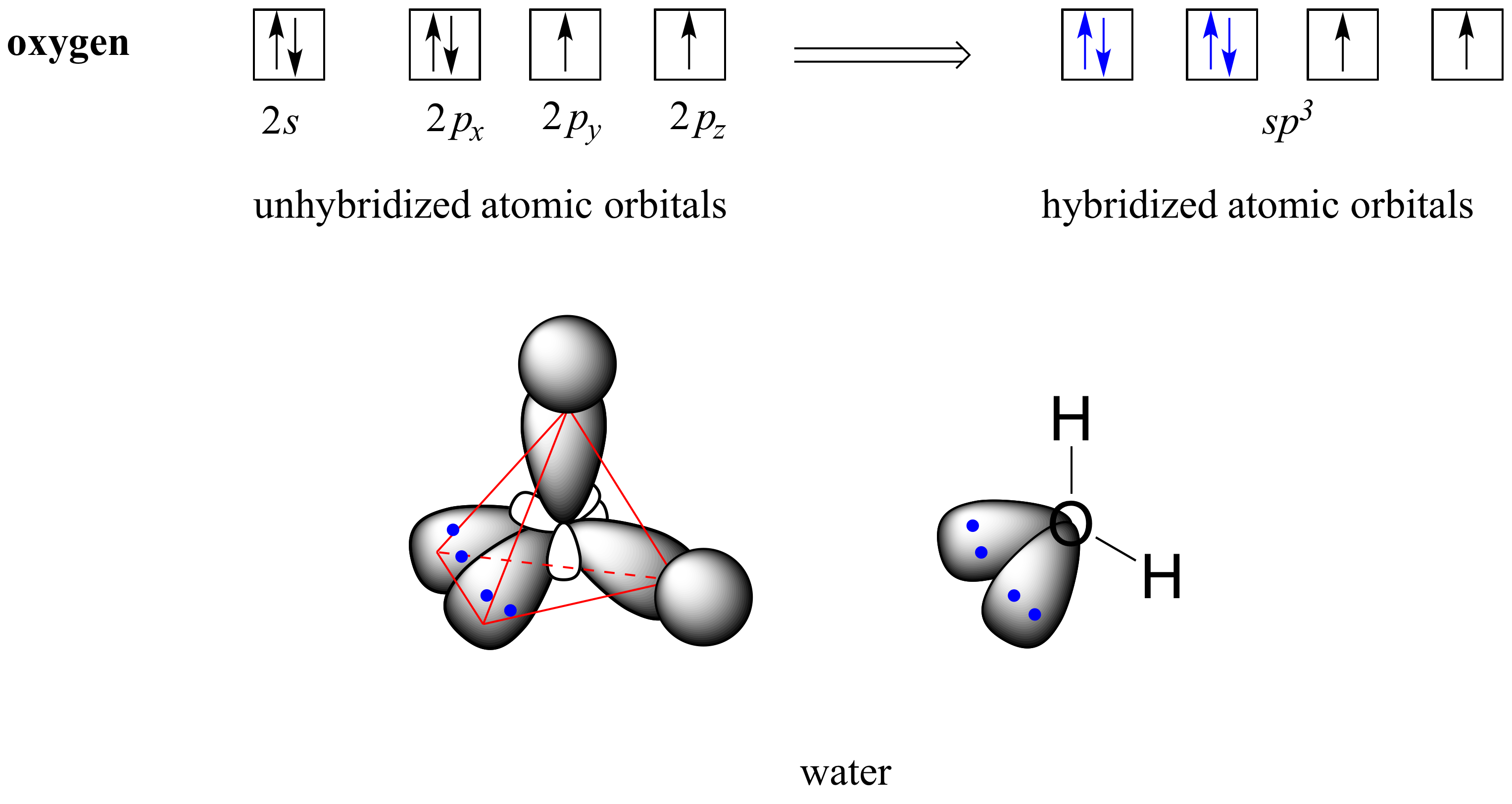
2.2 Hybrid orbitals Chemistry LibreTexts
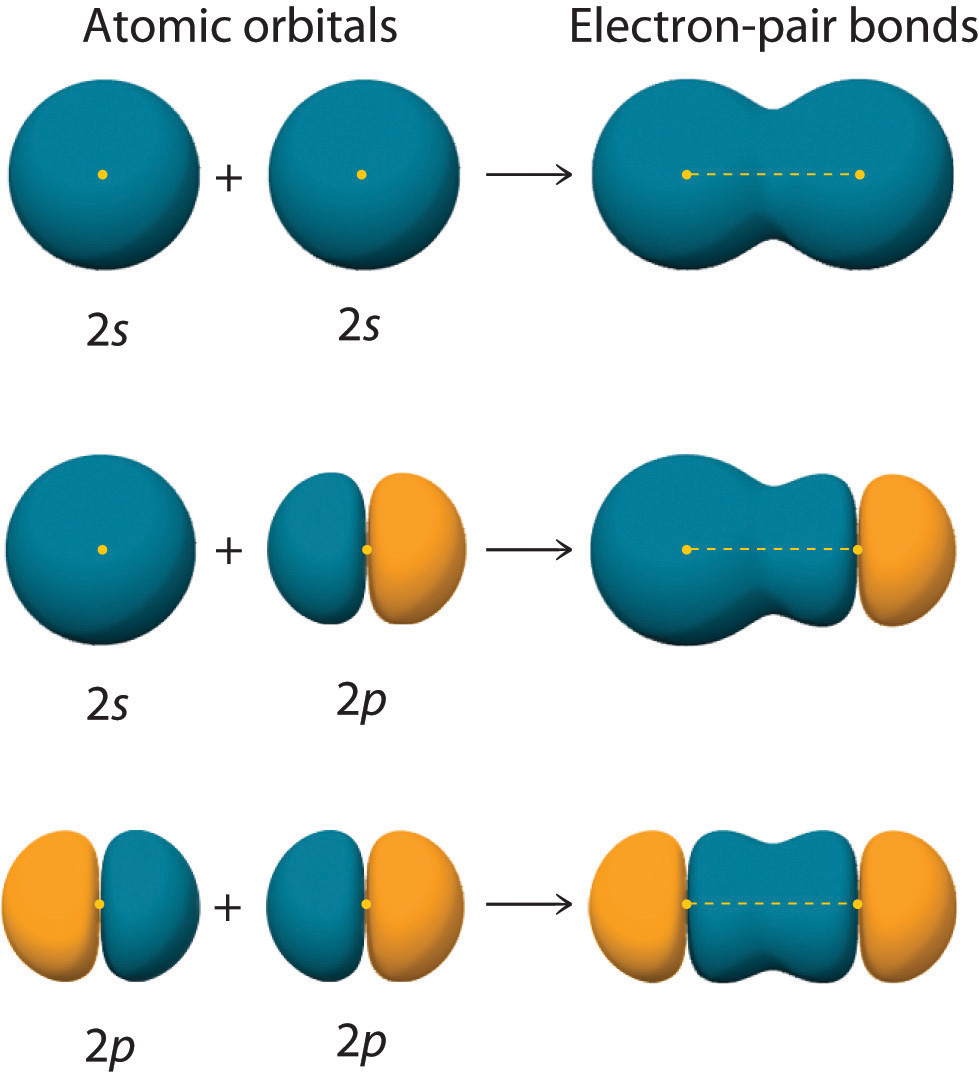
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
This Type Of Hybridization Is Required Whenever An Atom Is Surrounded By Four Groups Of Electrons.
Learn About Sp² Hybridized Orbitals And Pi Bonds.
Web A Set Of Hybrid Orbitals Is Generated By Combining Atomic Orbitals.
1.9M Views 3 Years Ago New Ap & General Chemistry Video Playlist.
Related Post: