Drawing Of Ammonia
Drawing Of Ammonia - Web draw the lewis diagram as below: Web you should consider the positions of the four atoms in ammonia to be essentially fixed in relation to each other. Number of electron regions in ammonia. Click on the structure to rotate it and view it from various angles. 31k views 4 years ago. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. Number of electron regions around nitrogen atom in ammonia. Web nitrogen fixation is a fundamental chemical process crucial for the ecosystem. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. It is clear to understand that the geometrical structure of nh3 will be bent. Web ammonia measurements are mainly of use in the diagnosis of urea cycle deficiencies (any neonate with unexplained nausea, vomiting, or neurological deterioration appearing after first feeding), and they play an important part in the detection of reye syndrome. The nh 3 chemical name is. Web. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. Web you should consider the positions of the four atoms in ammonia to be essentially fixed in relation to each other. Web an ammonia production facility in donaldsonville, louisiana. Click the structures to load the molecules. Related structures h 2 o | nh 3 |. Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. Ammonia is a colorless compound, used in making fertilizers. Web drawing the lewis structure for nh 3. Number of electron regions around nitrogen atom in ammonia. Chemistry in context december 2, 2019. Web nitrogen fixation is a fundamental chemical process crucial for the ecosystem. Web ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. Nh3. Click the structures to load the molecules. It is a stable hydride formed of one nitrogen and three hydrogen atoms. At least 80% of that yield serves as crop fertilizer. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Web learn the steps to draw the lewis structure of nh3 (ammonia) in just. We will walk through the steps used to construct the molecular orbital diagram of ammonia. It can form an nh4+ ion by accepting a proton. Web draw the lewis diagram as below: The nh 3 chemical name is. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Total electron region is taken by the summation of sigma bonds and lone pairs around relevant atom. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. How many bonding pairs are there around the n atom? Failed to load structure from its database. Number of electron regions around nitrogen atom in ammonia. Ammonia is a colorless gas with a chemical formula nh 3. It consists of hydrogen and nitrogen. Web ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). Web 155k views 3 years ago. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. We will walk through the steps used to construct the molecular orbital diagram of ammonia. Web 155k views 3 years ago. This problem has been solved! These are arranged in a tetrahedral shape. (ax 3 e 1 ). H • h • h • the lewis dot diagram helps to visualize the arrangement of electrons in a molecule and understand the bonding pattern. This is a clip from the complete video: It's not particularly difficult but is an important structure. These are arranged in a tetrahedral shape. Web ammonia measurements are mainly of use in the diagnosis of. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Draw a lewis structure for ammonia (nh3). Nh 3 (ammonia) is a commonly tested lewis structure. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. Draw a lewis structure for ammonia (nh3). How many bonding pairs are there around the n atom? Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Count the total number of valence electrons. (ax 3 e 1 ). In this video i draw the dot and cross diagram for nh3 (ammonia). Chemistry in context december 2, 2019. In the case of ammonia, the nitrogen atom shares its valence electrons with the hydrogen atoms, forming covalent bonds. For the nh3 structure use the periodic table to find the total number of valence electrons for the nh3 molecule. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. At least 80% of that yield serves as crop fertilizer. It's not particularly difficult but is an important structure.
Ammonia Formula
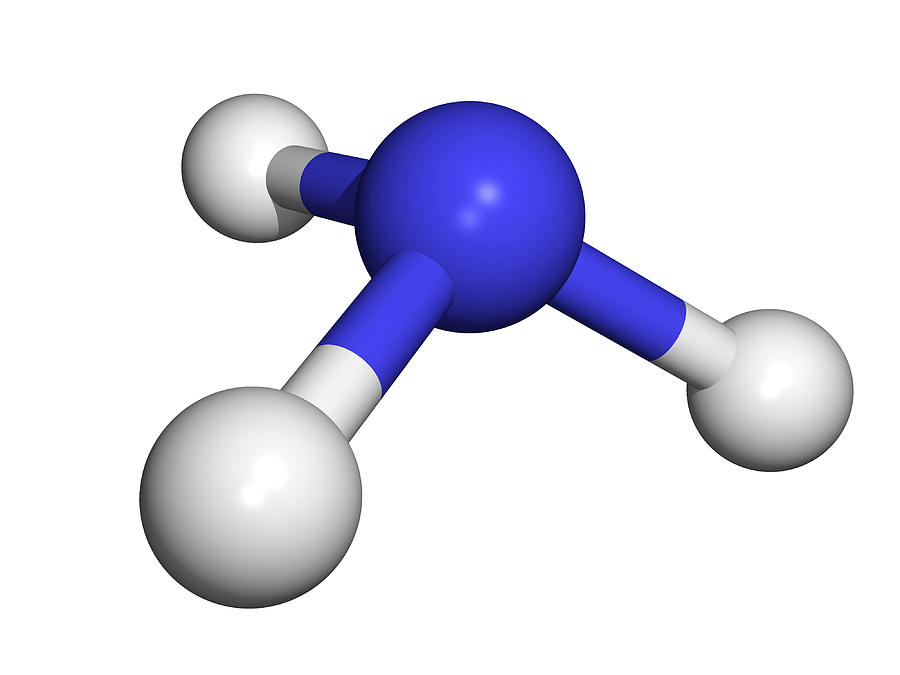
Structure Of Ammonia Molecule
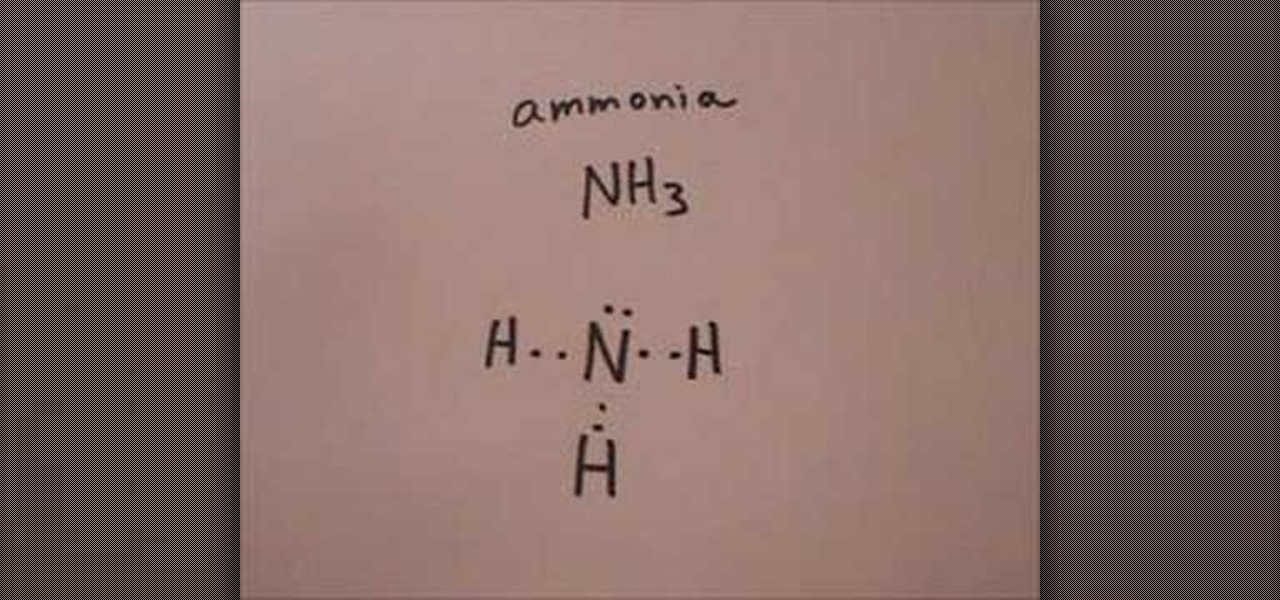
How to Draw the Lewis structure for ammonia « Science Experiments

Pictogram Van Ammonia Molecule Stock Illustratie Illustration of

Hand with pen drawing the chemical formula of ammonia Stock Photo Alamy
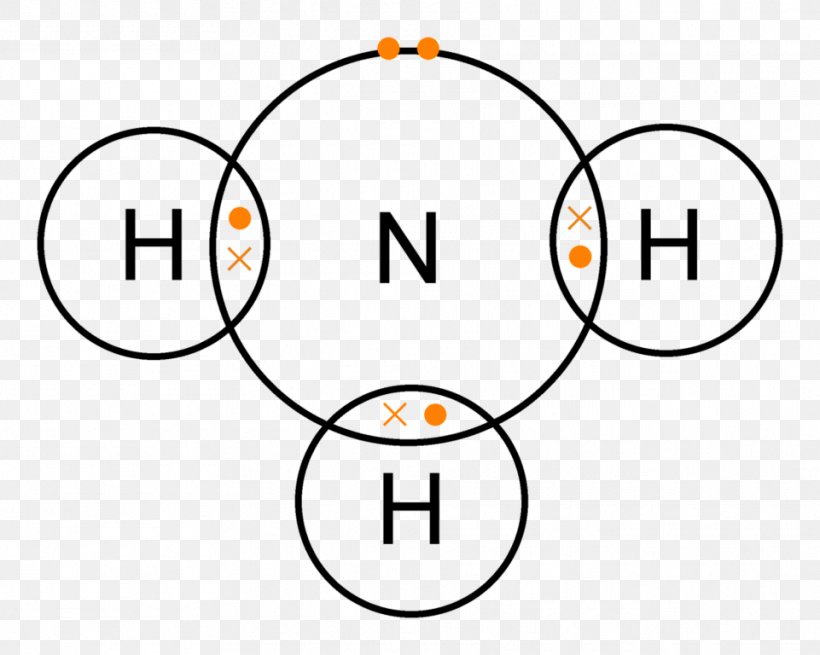
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG

3d render of molecular structure of ammonia isolated over white

Ammonia molecule model Stock Photo, Royalty Free Image 52584253 Alamy

Ammonia molecule, illustration Stock Image F030/7878 Science
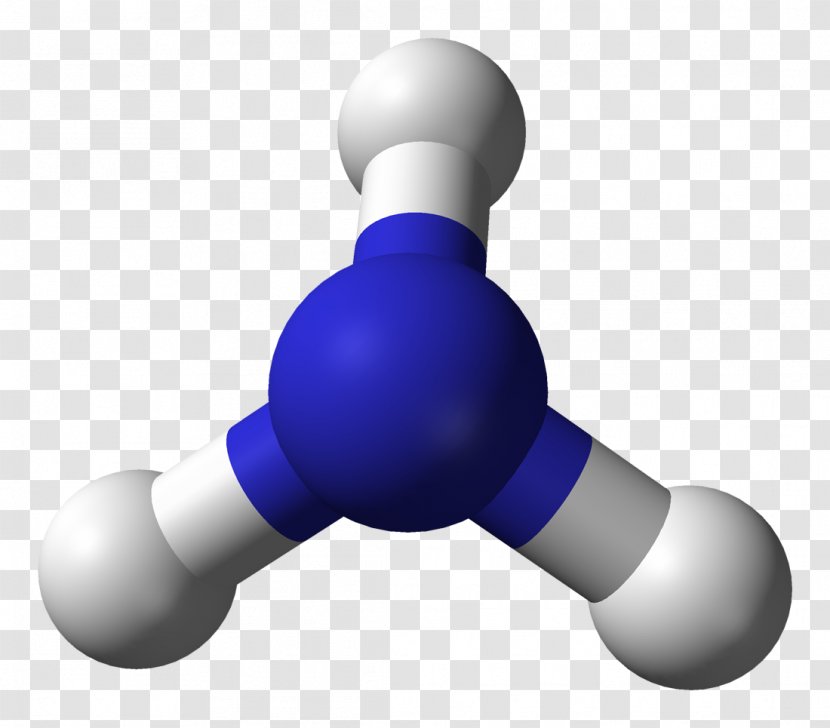
Ammonia Molecule Molecular Geometry Ballandstick Model Lewis
Web Draw The Lewis Diagram As Below:
31K Views 4 Years Ago.
Web Ammonia Has 4 Regions Of Electron Density Around The Central Nitrogen Atom (3 Bonds And One Lone Pair).
Web 155K Views 3 Years Ago.
Related Post: