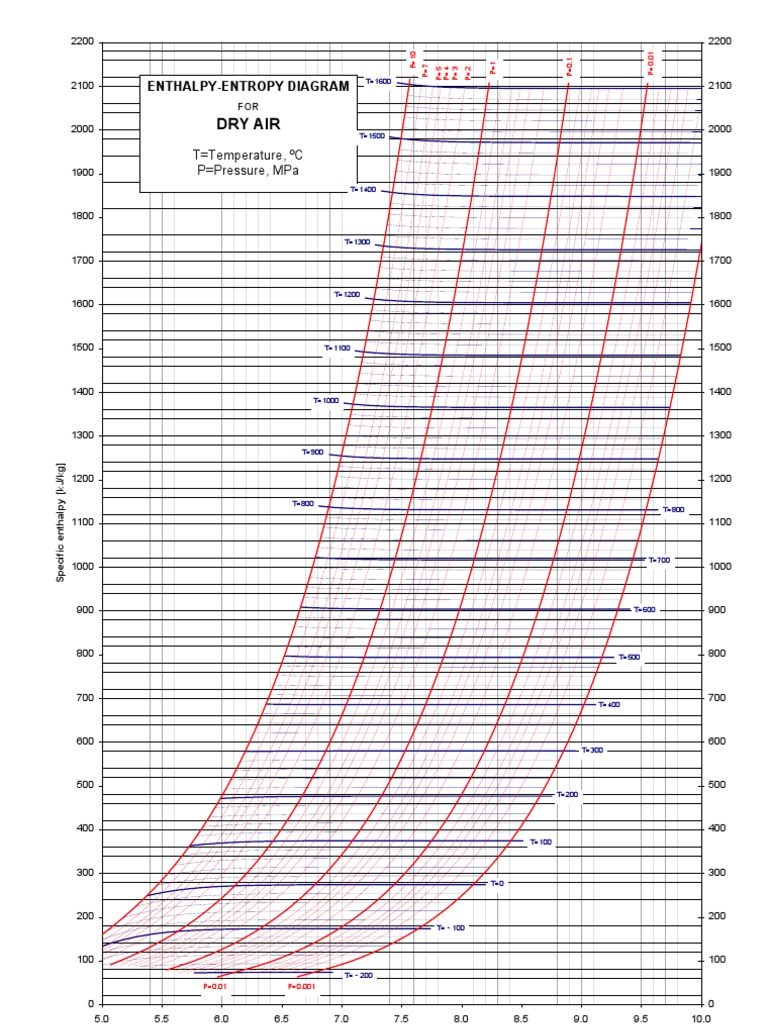
Entropy Enthalpy Chart
Entropy Enthalpy Chart - Major players in developing the second law. Denoted as δs δ s, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: Understanding enthalpy and entropy clarify what these differences mean and when to use each measurement. These lines extend at an angle from the saturated vapor line. A system will become more disordered, as time increases. Blue lines give (absolute) steam pressure;. Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 °c (212°f)). The values of the other related properties may be superimposed in the form of supplementary curves. Asked 8 years, 3 months ago. Select argon, benzene, or carbon dioxide with buttons. Click on diagram for properties. Web computation and uses of charts. Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. The values of the other related properties may be. Web standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01 96.2 Web for now, we will just look at enthalpy. Denoted as δs δ s, the change of entropy suggests that time itself is asymmetric with respect to order of an. A reaction is favored if the enthalpy of the. Select argon, benzene, or carbon dioxide with buttons. Major players in developing the second law. Click on diagram for properties. These lines extend at an angle from the saturated vapor line. These lines extend at an angle from the saturated vapor line. Web we know that the major difference between enthalpy and entropy is that even though they are part of a thermodynamic system, enthalpy is represented as the total heat content whereas entropy is the degree of disorder. Web the mollier diagram is a chart on which enthalpy (h) versus. Please allow more processing time for mixed refrigerant. In general, it is a relationship between enthalpy (measure of the energy of a thermodynamic system), air temperature, and moisture content. Most engineers understand the role units play in definition and verification of the engineering concepts, principles, equations and. Or input data for properties. Asked 8 years, 3 months ago. Web we know that the major difference between enthalpy and entropy is that even though they are part of a thermodynamic system, enthalpy is represented as the total heat content whereas entropy is the degree of disorder. A system will become more disordered, as time increases. Web standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Green lines show steam temperature; Click on diagram for properties. Web for now, we will just look at enthalpy. Denoted as δs δ s, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: A system will become more disordered, as time increases. Understanding enthalpy and entropy clarify what these differences mean and when to use each measurement. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well. These lines extend at an angle from the saturated vapor line. Web the thermodynamic arrow of time (entropy) is the measurement of disorder within a system. Please allow more processing time for mixed refrigerant. A system will become more disordered, as time increases. Click on diagram for properties. Major players in developing the second law. Enthalpy represents the total energy within a substance, however, it is impossible to directly measure. In a closed system, \ (\begin {array} {l}t.\delta s=\delta h\end {array} \) Denoted as δs δ s, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: Web where. In a closed system, \ (\begin {array} {l}t.\delta s=\delta h\end {array} \) Denoted as δs δ s, the change of entropy suggests that time itself is asymmetric with respect to order of an isolated system, meaning: Web the figures and tables below shows how water enthalpy and entropy changes with temperature (°c and °f) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 °c (212°f)). Enthalpy is the heat content of a system. These lines extend at an angle from the saturated vapor line. Isotherm, °c isentrop, kj/ (kg*k) quality. Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds. Web standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01 96.2 Web where enthalpy is a measurement of energy potential, entropy measures the randomness of energy with relation to heat. Enthalpy represents the total energy within a substance, however, it is impossible to directly measure. A reaction is favored if the enthalpy of the. Web the diagram below can be used to determine enthalpy versus entropy of water and steam. Web the mollier diagram is a chart on which enthalpy (h) versus entropy (s) is plotted. Please allow more processing time for mixed refrigerant. Web computation and uses of charts. Web the thermodynamic arrow of time (entropy) is the measurement of disorder within a system.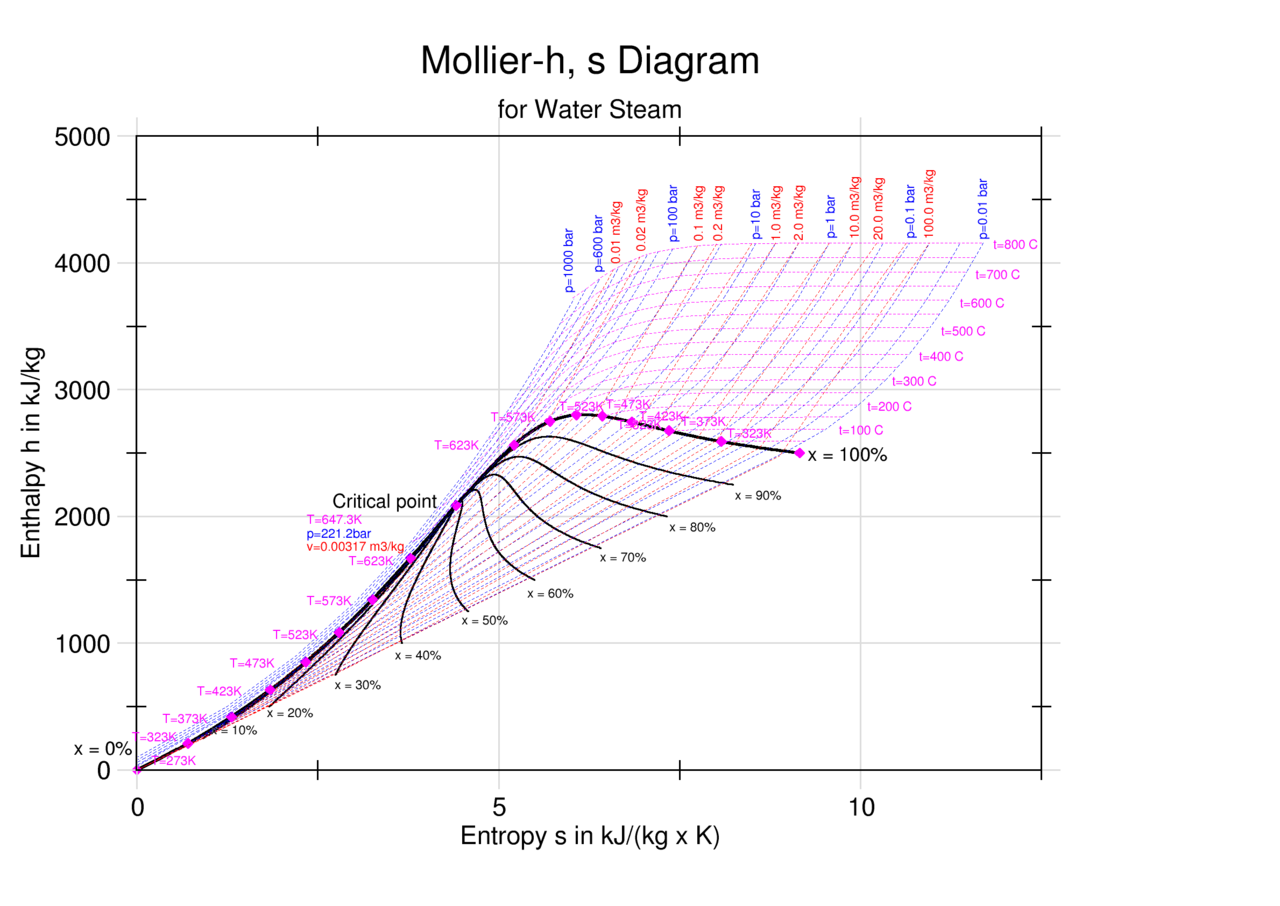
Enthalpy Entropy (hs) or Mollier Diagram

Entropy And Enthalpy Chart
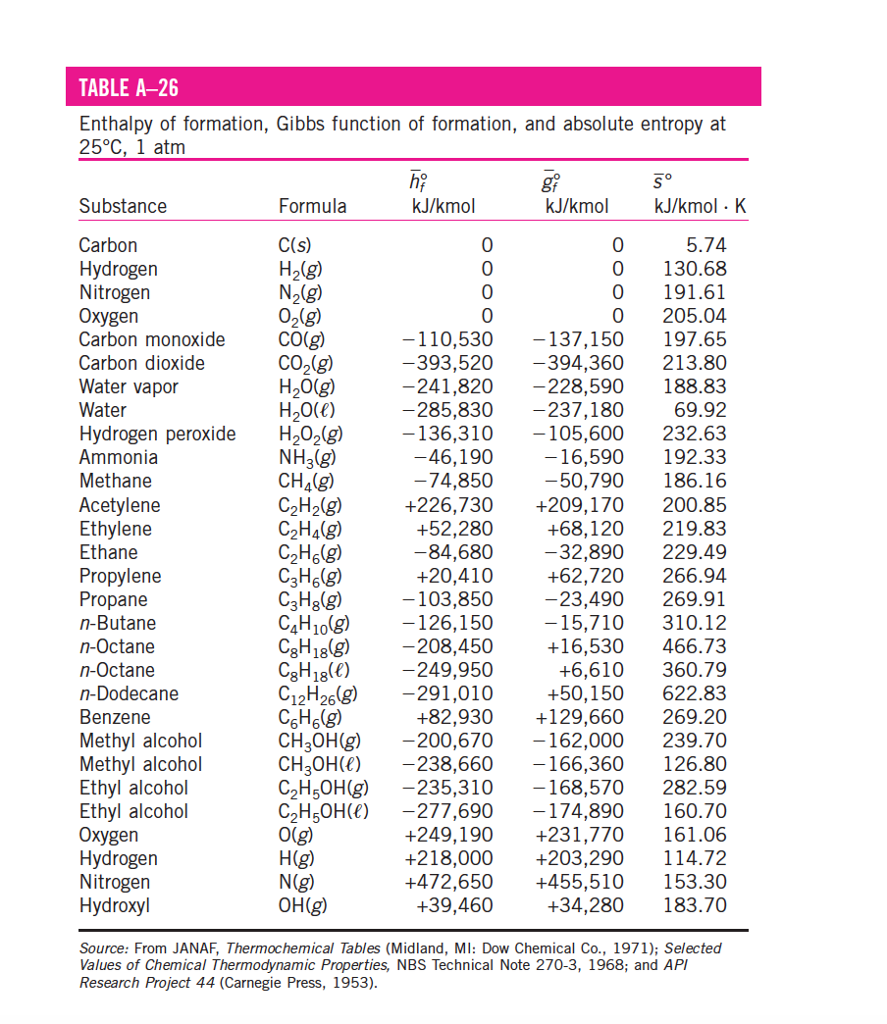
Standard Entropy Chart

The Mollier Or Specific Enthalpy Entropy Diagram Or C vrogue.co
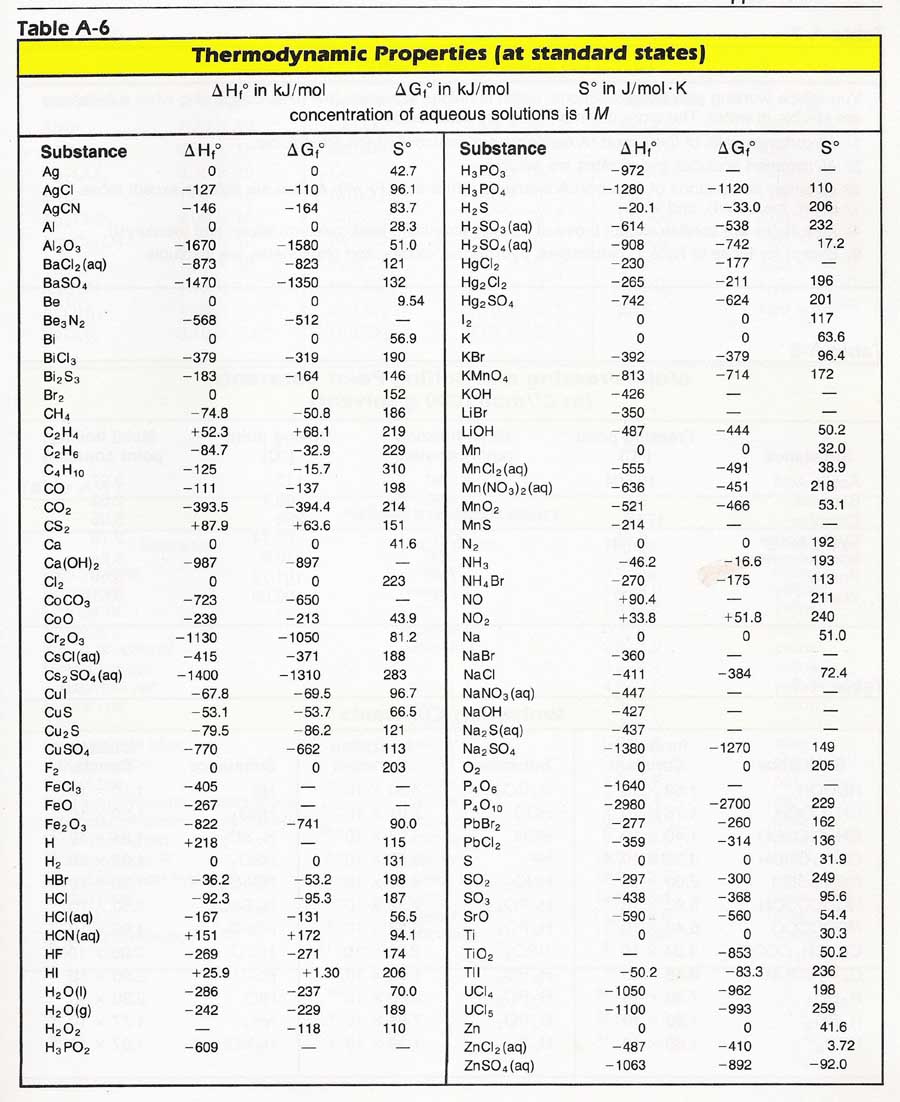
Entropy Table
Enthalpy And Entropy Chart
EnthalpyEntropy Diagram (Air) Enthalpy Statistical Mechanics
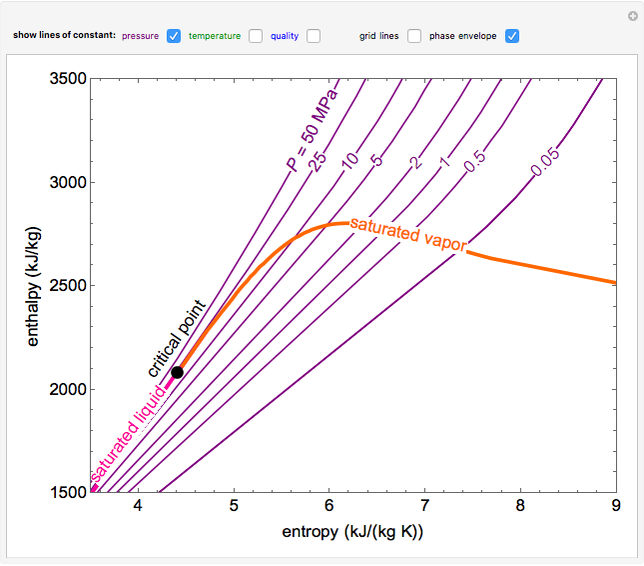
enthalpyentropydiagramforwater LearnChemE
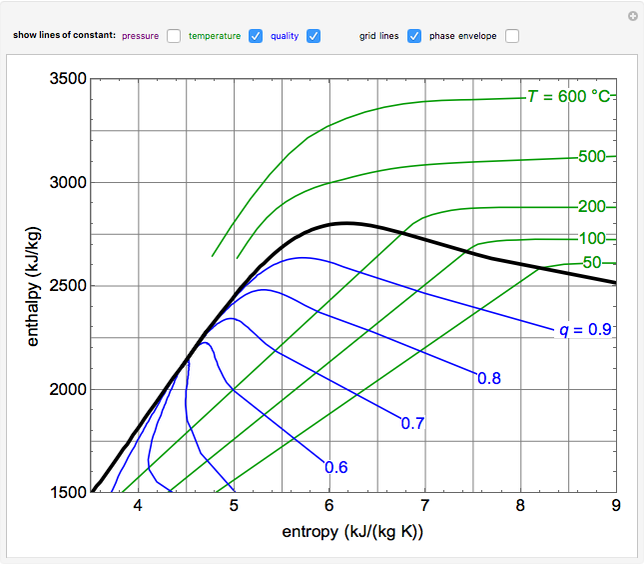
EnthalpyEntropy Diagram for Water Wolfram Demonstrations Project

Enthalpy And Entropy Chart
Most Engineers Understand The Role Units Play In Definition And Verification Of The Engineering Concepts, Principles, Equations And.
U = Specific Internal Energy.
Understanding Enthalpy And Entropy Clarify What These Differences Mean And When To Use Each Measurement.
Green Lines Show Steam Temperature;
Related Post: