Exothermic Reaction Chart
Exothermic Reaction Chart - Web in chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is an exothermic process. An endothermic reaction or process takes place when the system absorbs heat energy from the surrounding environment. When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. Web e x o t h e r m i c p r o c e s s. In a reaction, any reaction, the same general trend occurs. Some of this energy is given off as heat, and some does work pushing the piston in the cylinder. Web an exothermic reaction is a reaction in which energy is released in the form of light or heat. Web in thermochemistry, an exothermic reaction is a reaction for which the overall standard enthalpy change δ h ⚬ is negative. [1] [2] exothermic reactions usually release heat. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Such a reaction is said to be endothermic. Web in chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is an exothermic process. Web e x o t h e r m i c p r o c e s s. This is represented as dh on the. The substances involved in the reaction are the system, and the engine and. A reaction for which the overall standard gibbs energy change δ g ⚬ is negative. [2]. In endothermic reactions, the reactants have stronger bonds than the products. An explanation of this graph is as follows. The enthalpy change, δh, of an endothermic reaction is positive, because heat. Web e x o t h e r m i c p r o c e s s. Energy diagram for exothermic reaction. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. In an exothermic reaction or process, energy is released into the environment, usually in the form of. When a reaction absorbs or emits heat at constant pressure, the enthalpy changes. The thermometer shows the initial temperature, which is room temperature. When a chemical reaction occurs, energy is transferred to or from the surroundings. Web in chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is. Web in chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is an exothermic process. The thermometer shows the initial temperature, which is room temperature. The sign conventions for heat flow and enthalpy changes are summarized in. Web the reaction of gasoline and oxygen is exothermic. Energy diagram. If δh is negative, the process releases heat to the surroundings and is said to be exothermic. The sign conventions for heat flow and enthalpy changes are summarized in. Many chemical reactions release energy in the form of heat, light, or sound. In this investigation, students classify chemical reactions as exothermic or endothermic. In endothermic reactions, the reactants have stronger. Web e x o t h e r m i c p r o c e s s. It represents the heat content of a reaction. The sign conventions for heat flow and enthalpy changes are summarized in. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. There is. A chemical reaction always involves a change in energy. Exothermic reactions are reactions that release energy into the environment in the form of heat. An energy diagram represents this change. This information can be shown as. Web in chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is. Endothermic reactions take in energy and the temperature of the surroundings. What is an endothermic reaction? The beaker now contains sodium chloride and. This information can be shown as. This is represented as dh on the diagram. Web if δh is positive, the process absorbs heat from the surroundings and is said to be endothermic. It represents the heat content of a reaction. What is an exothermic reaction? Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Web in thermochemistry, an exothermic reaction is a reaction for which. Web in thermochemistry, an exothermic reaction is a reaction for which the overall standard enthalpy change δ h ⚬ is negative. [1] [2] exothermic reactions usually release heat. In a reaction, any reaction, the same general trend occurs. The enthalpy change, δh, of an endothermic reaction is positive, because heat is applied to the system. A chemical reaction always involves a change in energy. The sign conventions for heat flow and enthalpy changes are summarized in. An explanation of this graph is as follows. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Web the peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. Web an exothermic reaction is a reaction in which energy is released in the form of light or heat. Web an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. There is usually a temperature change. Web updated on september 12, 2019. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Web how can energy change. A reaction for which the overall standard gibbs energy change δ g ⚬ is negative. [2]. What is an endothermic reaction?
Exothermic and Endothermic Processes Introduction to Chemistry

(A) An exothermic reaction system during normal operation. (B
:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Endothermic and Exothermic Chemical Reactions

Exothermic energy diagram. MCAT Pinterest Diagram, Chemistry and
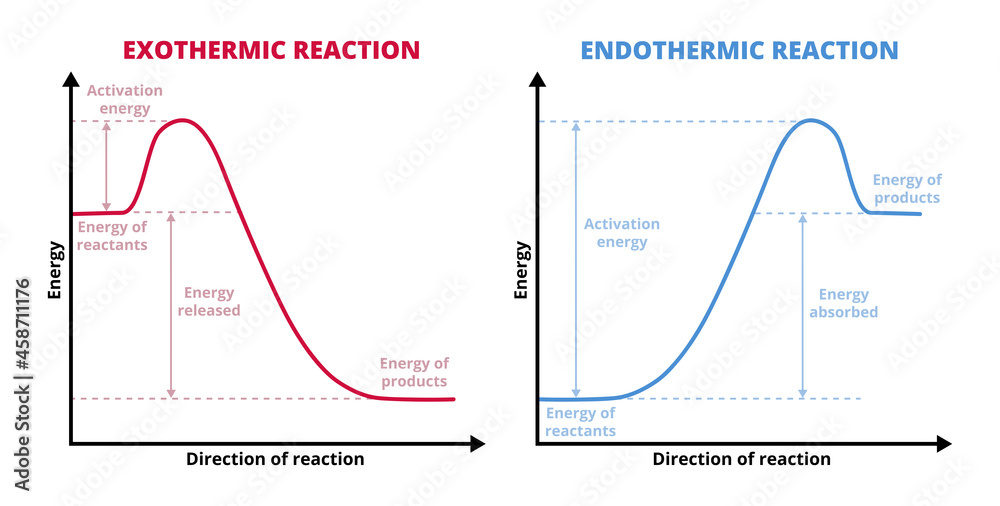
Vector graphs or charts of endothermic and exothermic reactions
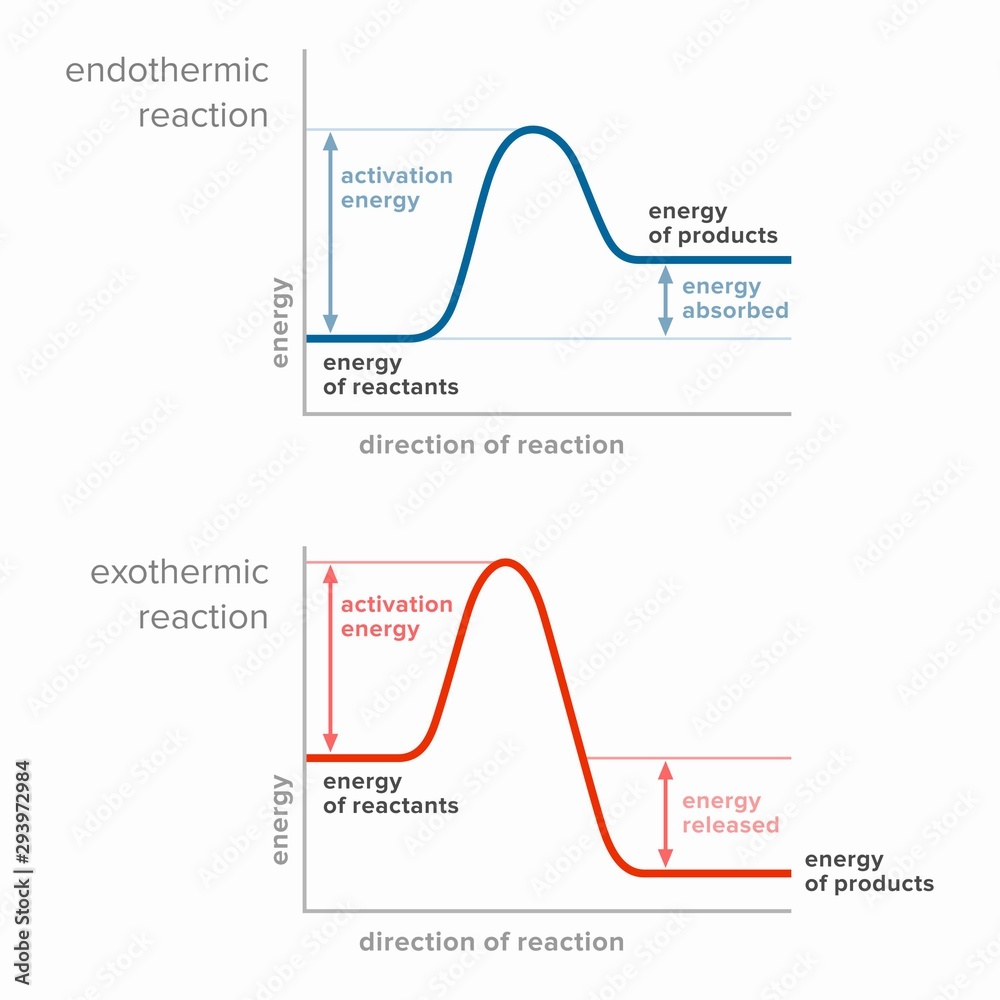
Activation energy in endothermic and exothermic reactions. Stock
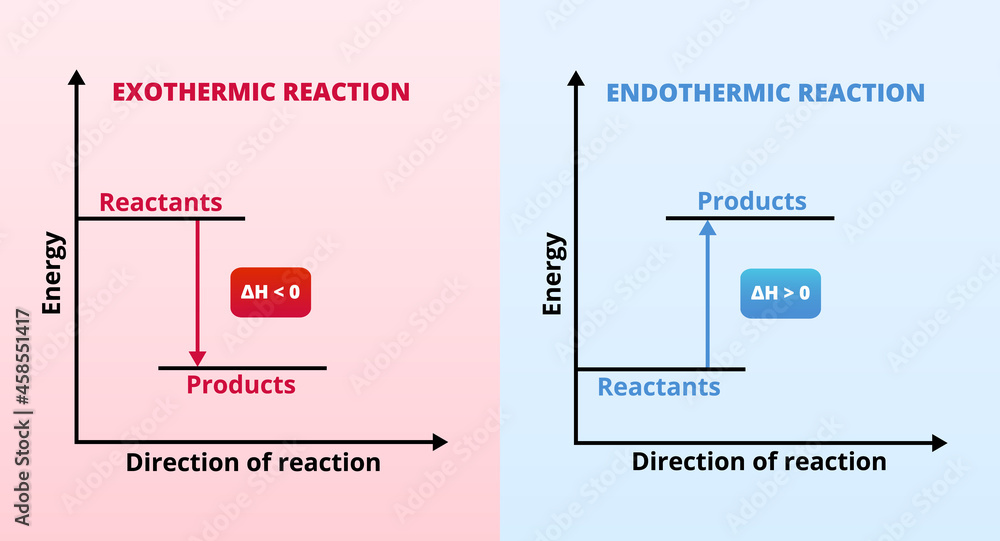
Vecteur Stock Vector graphs or charts of endothermic and exothermic
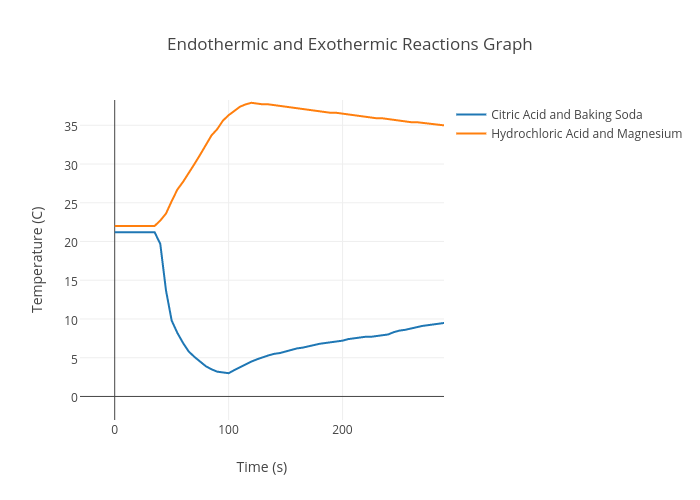
Endothermic and Exothermic Reactions Graph scatter chart made by
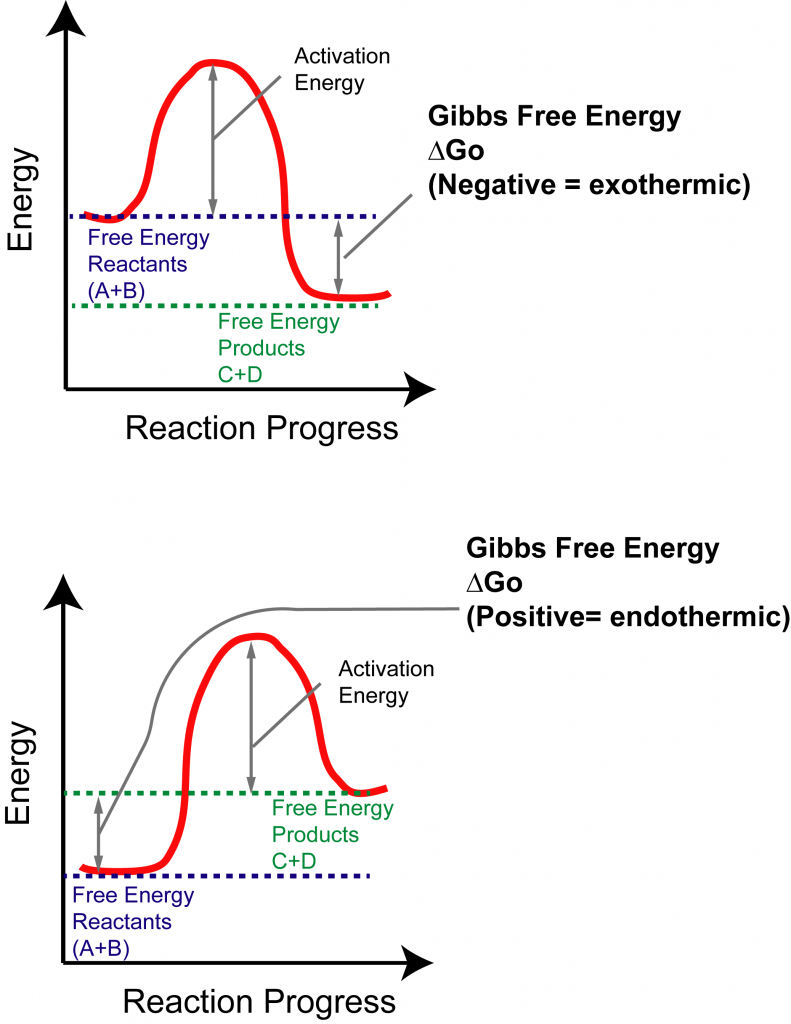
How to Interpret Thermodynamics of Reactions
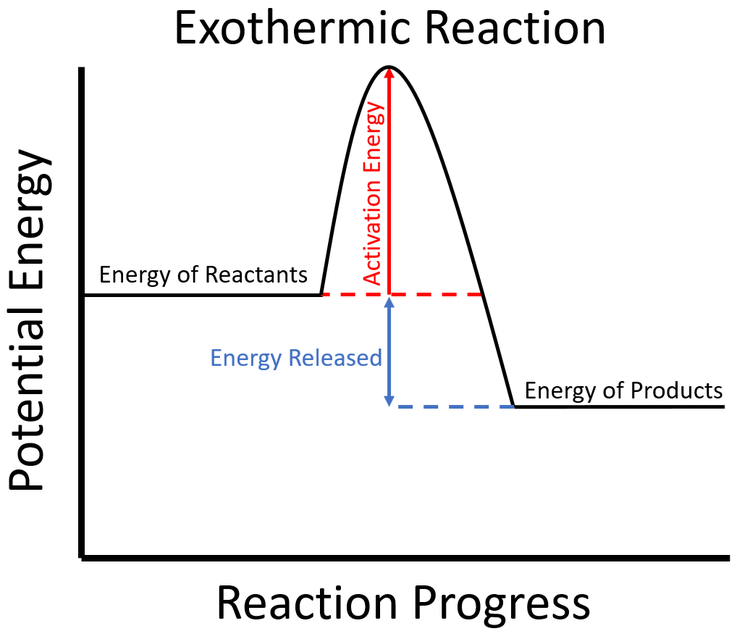
Exothermic Key Stage Wiki
In An Exothermic Reaction Or Process, Energy Is Released Into The Environment, Usually In The Form Of Heat, But Also Electricity, Sound, Or Light.
A Popular Example Of An Endothermic Chemical Reaction Is Photosynthesis.
If Δh Is Negative, The Process Releases Heat To The Surroundings And Is Said To Be Exothermic.
Web In Chemical Reactions, Bond Breaking Requires An Input Of Energy And Is Therefore An Endothermic Process, Whereas Bond Making Releases Energy, Which Is An Exothermic Process.
Related Post: