Free Energy Of Formation Chart
Free Energy Of Formation Chart - Δg can predict the direction of the chemical reaction under two conditions: These values are valid for the temperature 25 c. Where h is enthalpy, t is temperature (in kelvin, k ), and s is the entropy. When δg° < 0, the process is thermodynamically favored. Gibbs free energy = g = h − ts. When a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: What is the heat absorbed or released when 1 mole of a substance is formed from its respective elements in their standard states? Temperatures where either the metal or oxide melt or vaporize are marked on the diagram. Its symbol is δ f g˚. The standard gibbs free energy of formation at 25°c (298,15 k) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their standard state (stable forms at 1 bar and 25°c) s°: Learn with 13 free energy of formation flashcards in the free vaia app. Bromides chlorides fluorides hydrides iodides nitrides oxides sulfides selenides tellurides. Web gibbs free energy and spontaneity. Web free energy of formation of binary compounds: Web free energy of formation is negative for most metal oxides, and so the diagram is drawn with ∆g=0 at the top of. What is the heat absorbed or released when 1 mole of a substance is formed from its respective elements in their standard states? All standard state, 25 °c and 1 bar (written to 1 decimal place). Δg can predict the direction of the chemical reaction under two conditions: When δg° < 0, the process is thermodynamically favored. When a process. If you have already read the page about how to do this with total entropy changes, you will find a little bit of repetition on this page. Web introduction to gibbs free energy (video) | khan academy. Web standard heats and free energies of formation and absolute entropies of elements and inorganic compounds. These values are valid for the temperature. Δg can predict the direction of the chemical reaction under two conditions: Web gibbs free energy and spontaneity. Where h is enthalpy, t is temperature (in kelvin, k ), and s is the entropy. Web standard heats and free energies of formation and absolute entropies of elements and inorganic compounds. An atlas of charts for high‐temperature chemical calculations. When δg° < 0, the process is thermodynamically favored. Explain how temperature affects the spontaneity of some processes. A pure element in its standard state has a standard free energy of formation of zero. Δg can predict the direction of the chemical reaction under two conditions: Where h is enthalpy, t is temperature (in kelvin, k ), and s is. Its symbol is δ f g˚. Web standard free energies of formation. When a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: Δg can predict the direction of the chemical reaction under two conditions: Web introduction to gibbs free energy. Web definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds. Web the standard gibbs free energy of formation of a compound is the change of gibbs free energy that accompanies. Bromides chlorides fluorides hydrides iodides nitrides oxides sulfides selenides tellurides. The standard gibbs free energy of formation at 25°c (298,15 k) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their standard state (stable forms at 1 bar and 25°c) s°: The standard gibbs free energy change, δg°, indicates the. Web introduction to gibbs free energy (video) | khan academy. Web standard gibb's energies of formation for. Web the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states. Web free energy of formation is negative for most metal oxides, and so the diagram is drawn with ∆g=0 at the top of the diagram, and the values of ∆g shown are all negative numbers. Do the results indicate the reaction to be spontaneous or nonspontaneous under standard conditions? What is the heat absorbed or released when 1 mole of. The standard gibbs free energy of formation at 25°c (298,15 k) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their standard state (stable forms at 1 bar and 25°c) s°: Temperatures where either the metal or oxide melt or vaporize are marked on the diagram. Web standard gibb's energies of formation for. The standard entropy for 1 mol of the substance in its given state (g= gas and l= liquide) at 1 bar and 25°c. The figures include nomographs for equilibrium partial pressures. Web free energy of formation is negative for most metal oxides, and so the diagram is drawn with ∆g=0 at the top of the diagram, and the values of ∆g shown are all negative numbers. Name δh f ° (kj/mol) δg f ° (kj/mol) s° (j/mol k) h 2 (g) 0: Reed and julius klerer 1972 j. Web introduction to gibbs free energy (video) | khan academy. This page introduces gibbs free energy (often just called free energy), and shows how it can be used to predict the feasibility of reactions. These values are valid for the temperature 25 c. When a process occurs at constant temperature t and pressure p , we can rearrange the second law of thermodynamics and define a new quantity known as gibbs free energy: Journal of the electrochemical society , volume 119 , number 12 citation t. Explain how temperature affects the spontaneity of some processes. Web calculate the standard free energy change at room temperature, using (a) standard free energies of formation and (b) standard enthalpies of formation and standard entropies. Web the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states (the most stable form of the element at 1 bar of pressure and the specified temperature, usually 298.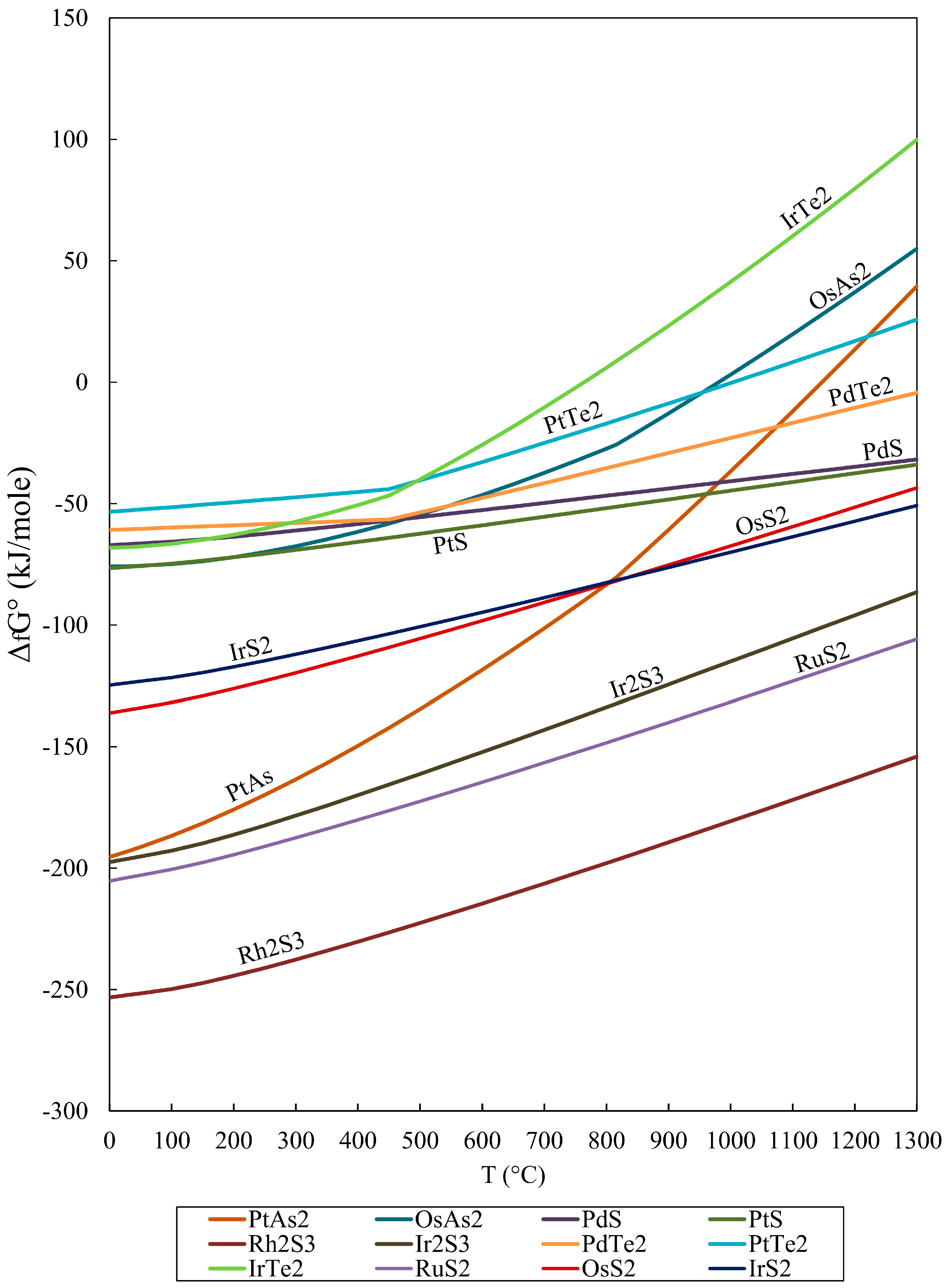
Standard Gibbs Free Energy Of Formation Table slideshare
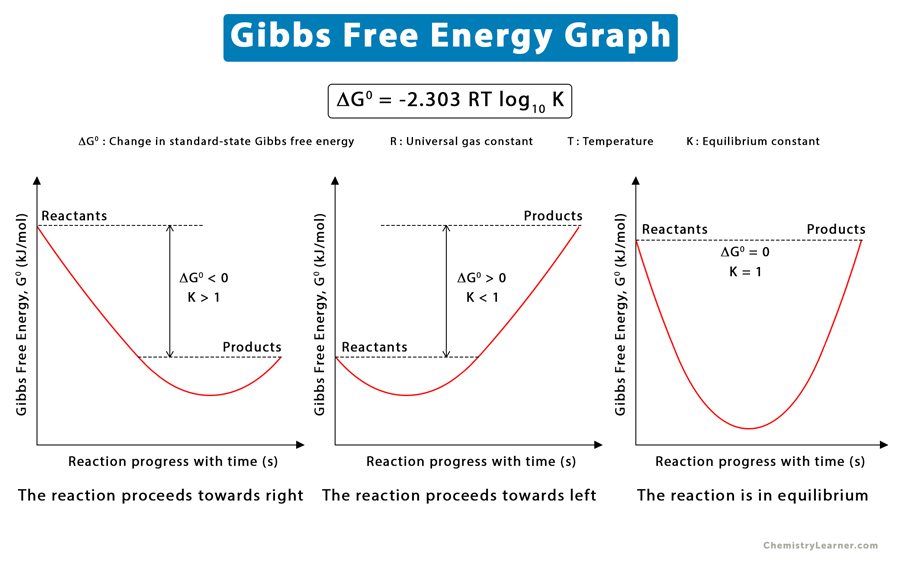
Gibbs Free Energy Definition, Equation, Unit, and Example
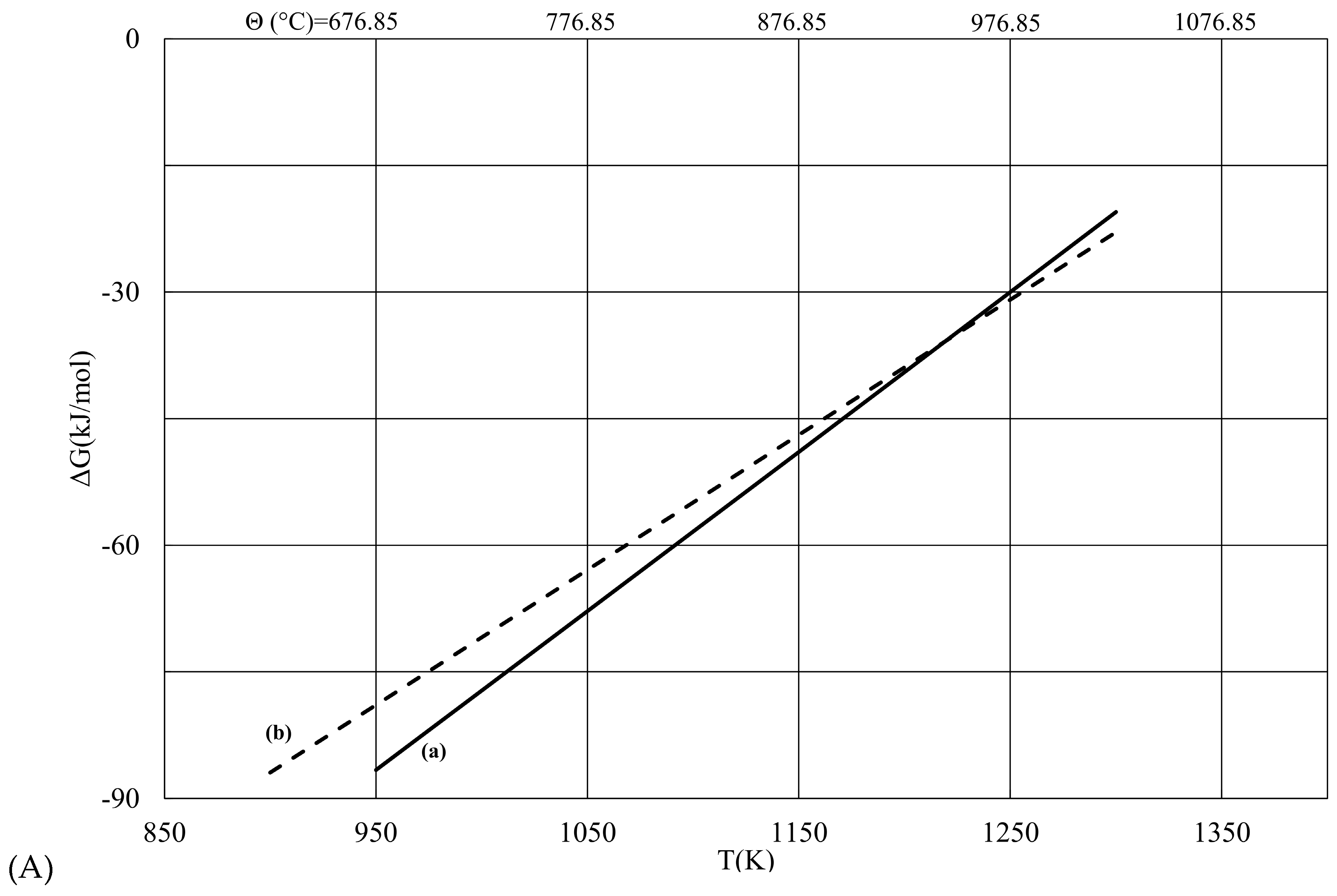
Geosciences Free FullText Gibbs Free Energy of Formation for

Standard Gibbs free energy of formation, standard enthalpy of formation
Standard Gibbs Free Energy Of Formation Table slideshare

Standard Gibbs Free Energy Of Formation Table slideshare
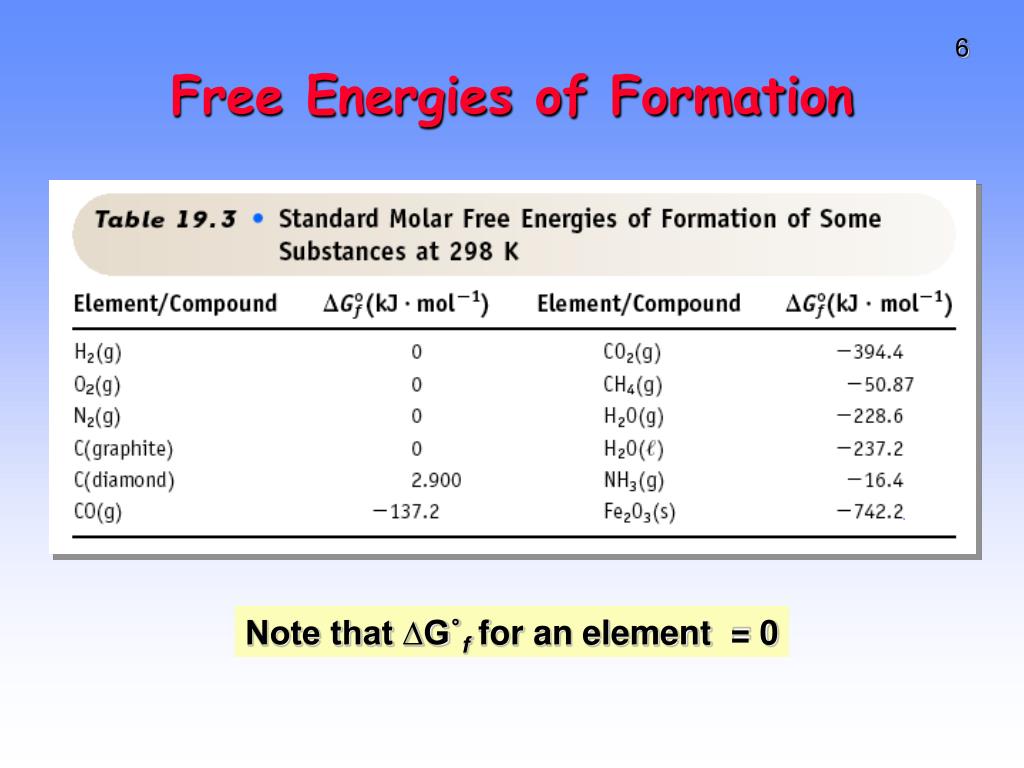
PPT Gibbs Free Energy, G PowerPoint Presentation, free download ID
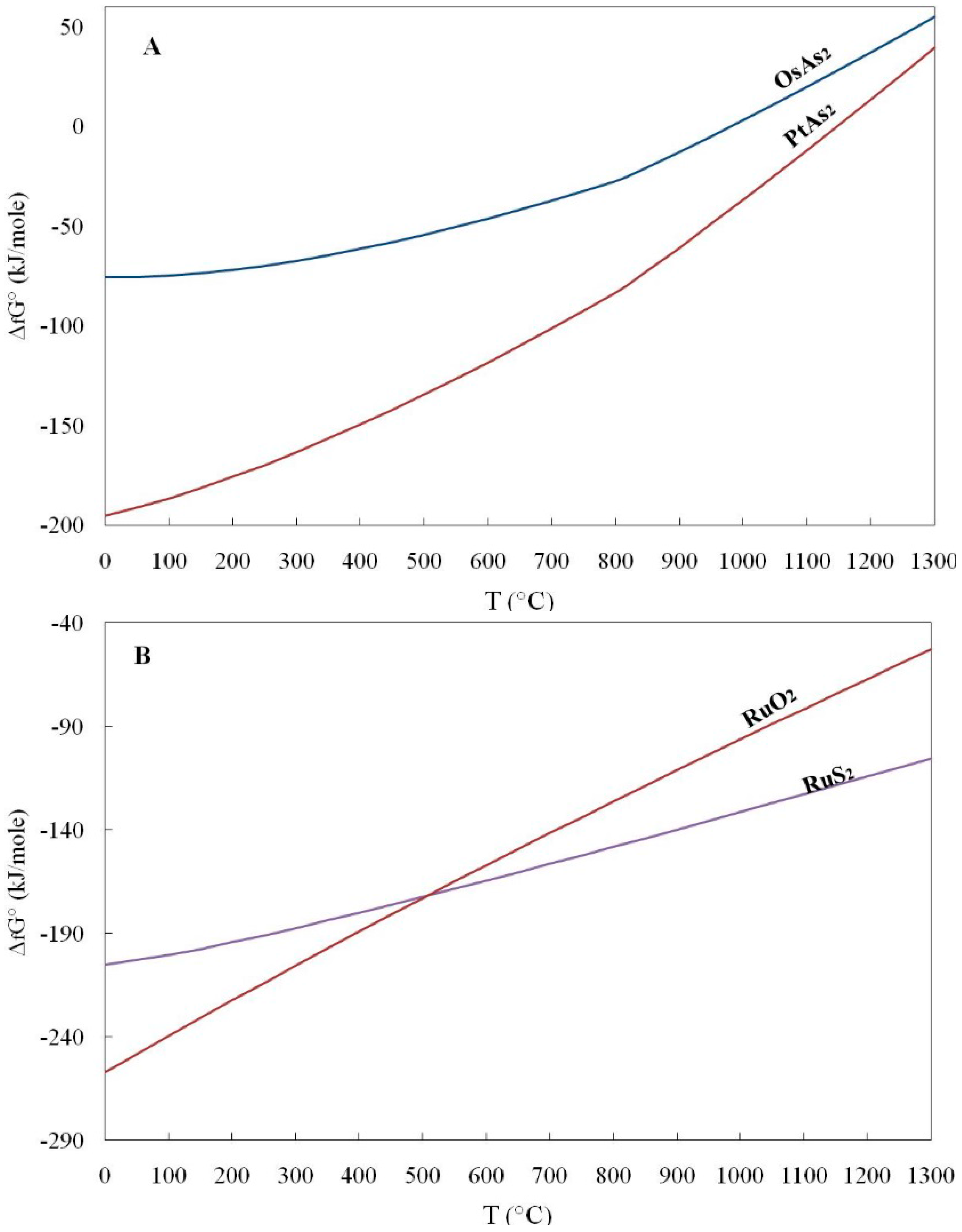
![[PDF] Gibbs Free Energy of Formation for Selected Platinum Group](https://d3i71xaburhd42.cloudfront.net/1dbf18616943ea1a81a4ada05098657bbd1510ba/2-Table1-1.png)
[PDF] Gibbs Free Energy of Formation for Selected Platinum Group

Gibbs free energy of formation for selected chemicals. The point [5

Gibbs free energy of formation values for various oxides. Download
Web Definition And Explanation Of The Terms Standard State And Standard Enthalpy Of Formation, With Listing Of Values For Standard Enthalpy And Gibbs Free Energy Of Formation, As Well As Standard Entropy And Molar Heat Capacity, Of 370 Inorganic Compounds.
Web Free Energy Of Formation Of Binary Compounds:
Web The Standard Gibbs Free Energy Of Formation Of A Compound Is The Change Of Gibbs Free Energy That Accompanies The Formation Of 1 Mole Of That Substance From Its Component Elements, In Their Standard States (The Most Stable Form Of The Element At 25 °C And 100 Kpa).
Learn With 13 Free Energy Of Formation Flashcards In The Free Vaia App.
Related Post: