Galvanic Action Chart
Galvanic Action Chart - First there must be two electrochemically dissimilar metals present. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Though the order of metals in a galvanic series remains the same in most conducting solutions, some. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. Tabular representation of the galvanic series. Yet in today’s construction world it can be easy for a contractor to mix the wrong type of fastener with the wrong metal. What are the causes of galvanic corrosion? This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Below is a galvanic reaction chart for dissimilar metals. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web the galvanic action increases as the metals are farther apart in the galvanic series. The list begins with the more active (anodic) metal and proceeds down. The metallic phases, compounds, dilution or enrichment regions of component elements, and oxidation films in the alloy showing different electrode potentials may also be galvanically with the metal, and deactivation and concentration effects. The list begins with the more active (anodic) metal and proceeds down. What is the galvanic series? If you're a designer working with exterior metals, you've probably heard of galvanic corrosion. In certain cases one metal immediately following another may be very corrosive. The closer together the material are on the chart to the right, the less galvanic action will. Web by simply choosing metals that avoid galvanic action there will never be an issue. In certain cases one metal immediately following another may be very corrosive. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. When dissimilar metals are used together in the. What are the causes of galvanic corrosion? The closer together the material are on the chart to the right, the less galvanic action will. If that’s not possible, other recommendations are: The following galvanic table lists metals in the order of their relative activity in seawater environment. Galvanic voltages relative to gold. Simpletwig will then try to. When is stainless steel passive or active. Though the order of metals in a galvanic series remains the same in most conducting solutions, some. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web this slide includes a chart of galvanic corrosion potential between common construction metals. This form of corrosion has the potential to attack junctions of metals, or regions where one construction metal contacts another. Web the galvanic action increases as the metals are farther apart in the galvanic series. We also provide other helpful methods for avoiding galvanic corrosion. Web this slide includes a chart of galvanic corrosion potential between common construction metals. When. It is not always true that there is greater corrosion the further down the scale one goes. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. Web fastened of galvanic corrosion in joint, it’s recommended to choose materials that are grouped together in. Tabular representation of the galvanic series. Types of galvanic corrosion in different metals and their alloys. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with a return current path. Below is a galvanic reaction chart for dissimilar metals. Web when dissimilar metals are connected — either by simple contact. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with. Most architects know enough about it to be dangerous, but what exactly causes this breakdown? To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the contact metal listed in the. The closer together the material are on the chart to the right, the less galvanic action will. Note that. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Values given here are for indicative purpose only. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the contact metal listed in the. Web fastened of galvanic corrosion in joint, it’s recommended to choose materials that are grouped together in the galvanic series chart. Web the galvanic action increases as the metals are farther apart in the galvanic series. Web by simply choosing metals that avoid galvanic action there will never be an issue. When is stainless steel passive or active. Yet in today’s construction world it can be easy for a contractor to mix the wrong type of fastener with the wrong metal. We also provide other helpful methods for avoiding galvanic corrosion. Simpletwig will then try to. Though the order of metals in a galvanic series remains the same in most conducting solutions, some. Note that there are several tables presented on this page and that they do not all agree. In certain cases one metal immediately following another may be very corrosive. You can also learn more about overcoming potentially compatibility issues between metals. In this respect, one should understand how to read the following chart which lists all metals. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with a return current path.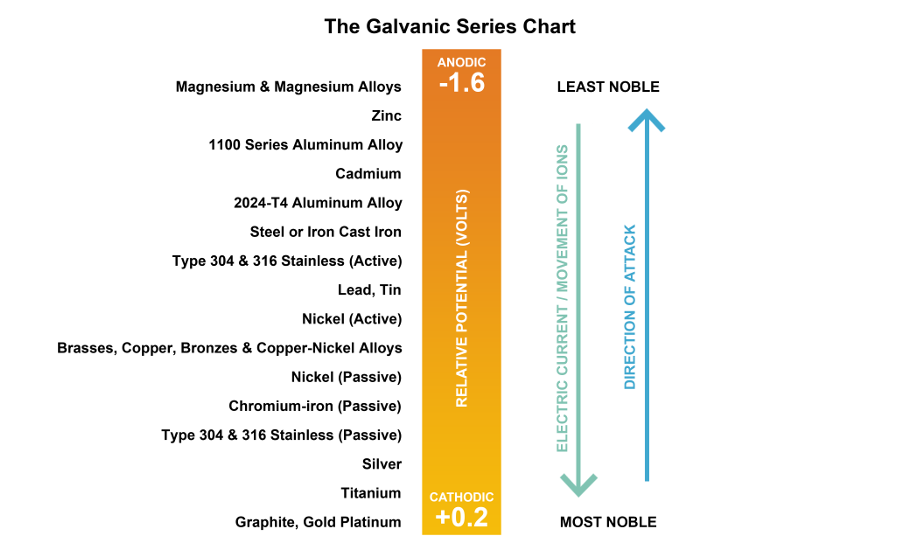
Galvanic Chart Of Metals
Galvanic Action Chart PDF
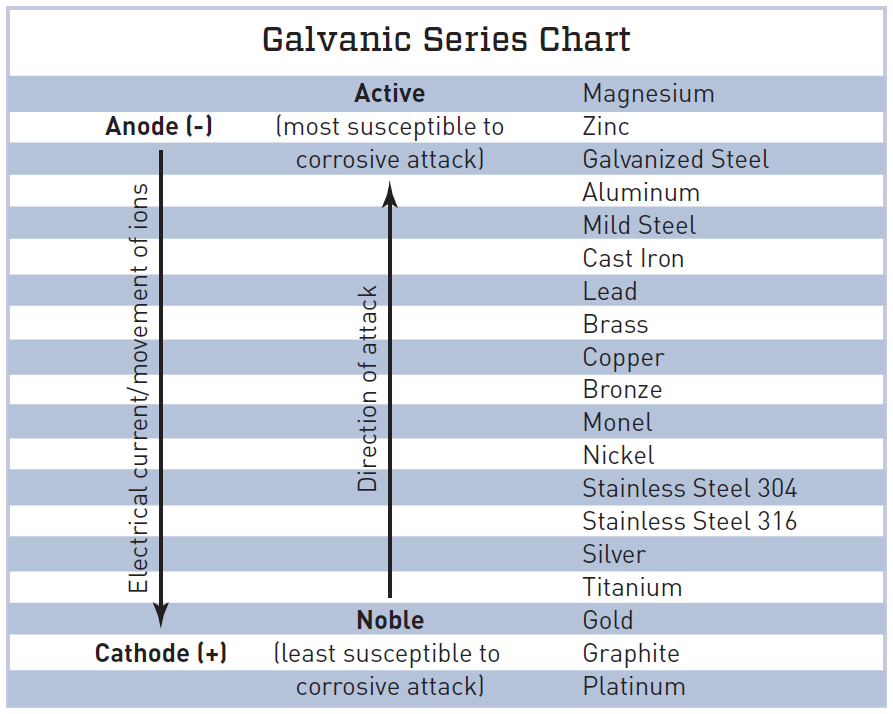
Galvanic Series Chart
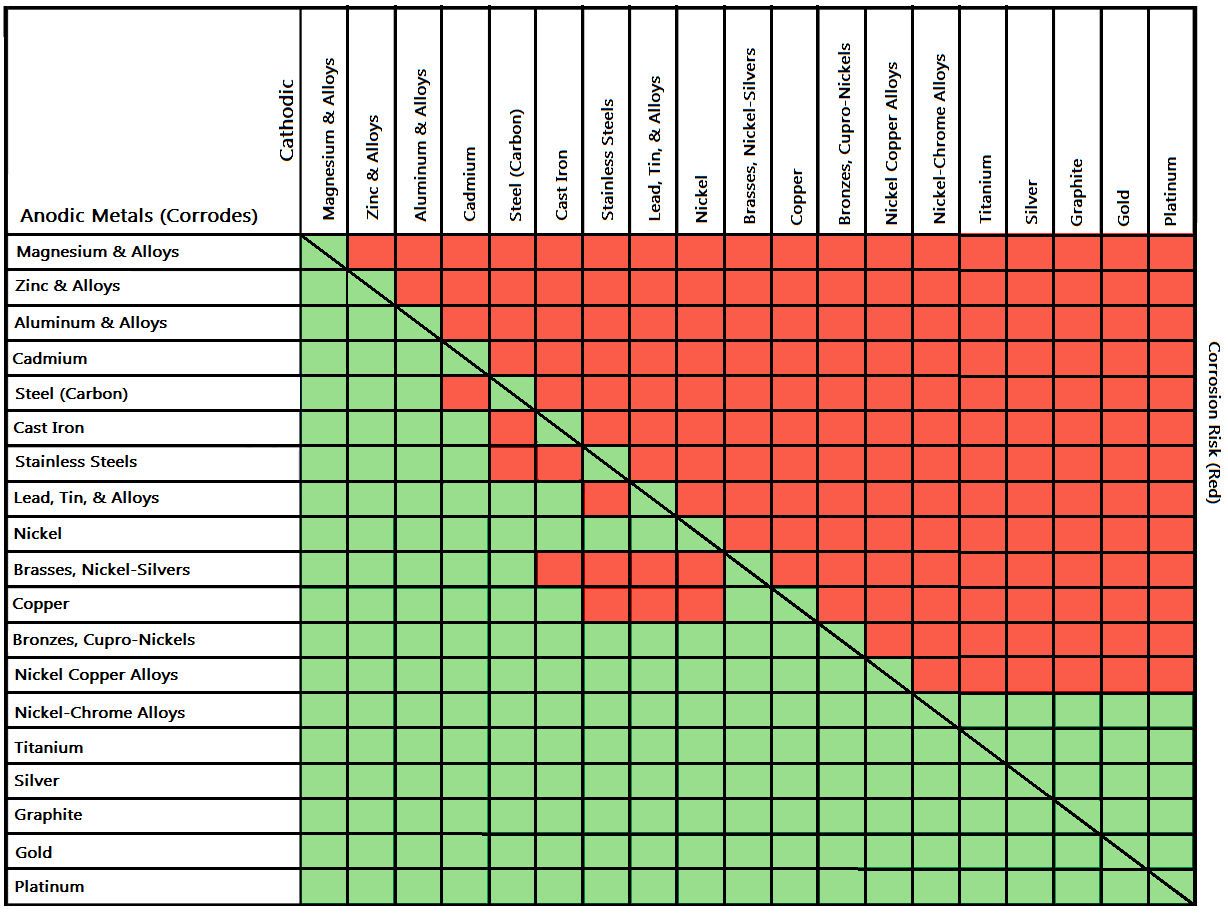
Galvanic Corrosion Common Questions Answered

Galvanic Reaction Chart All Points Fasteners
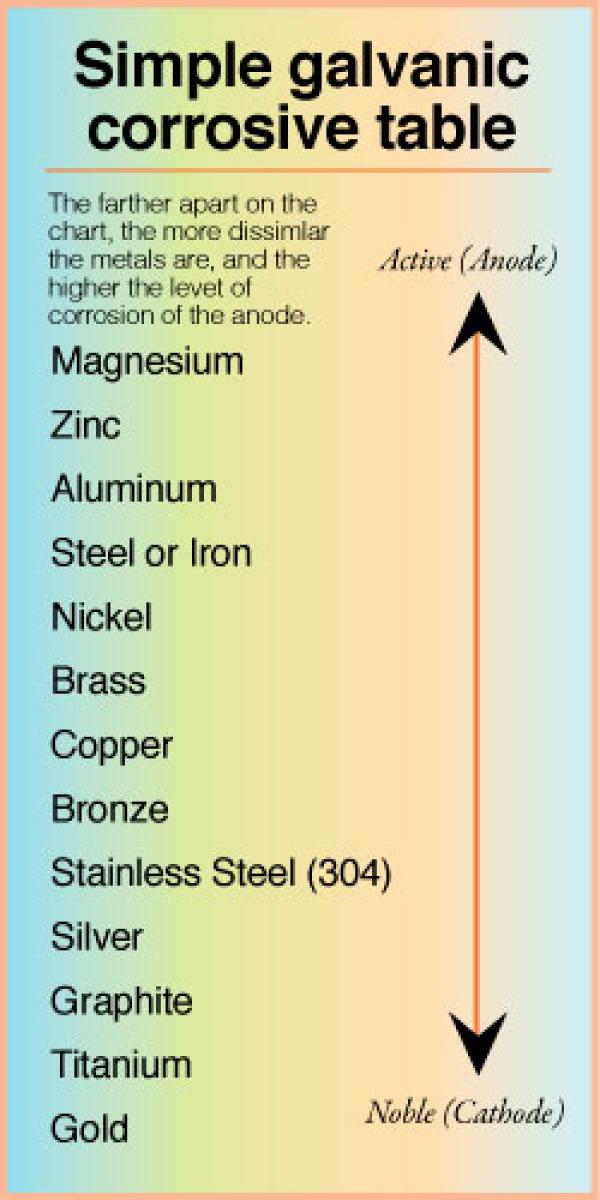
Galvanic Corrosion Chart Metals

Galvanic Action Corrosion Prevention Architect's Blog
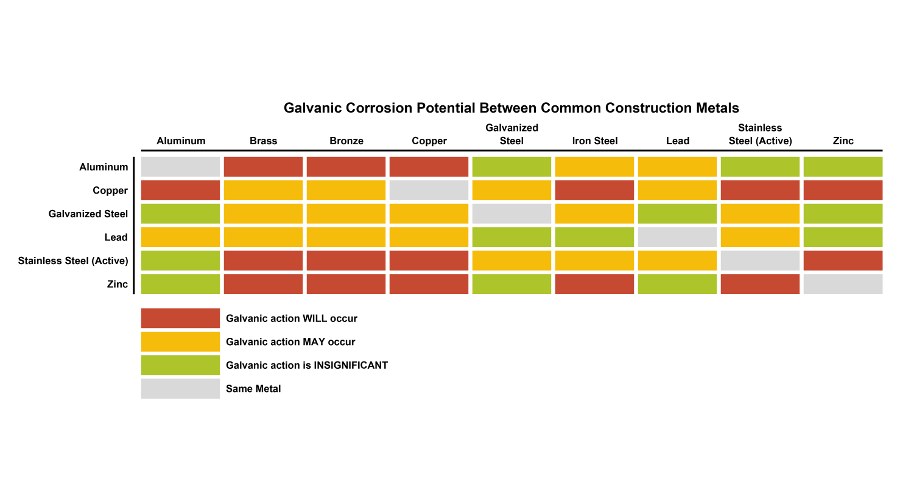
Galvanic Corrosion Compatibility Chart

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot

Prevent Galvanic Action Frame Building News
Select Materials That Are As Close Together As Possible In The Galvanic Series Chart.
The Below Galvanic Corrion Chart Or Anodic Index Table Shows Anodic Index For Different Materials.
When Dissimilar Metals Are Used Together In The Presence Of An Electrolyte, Separate Them With A Dielectric Material Such As Insulation, Paint Or Similar Surface Coating.
Web Below Is A Galvanic Reaction Chart For Dissimilar Metals.
Related Post:
