Galvanic Chart For Metals
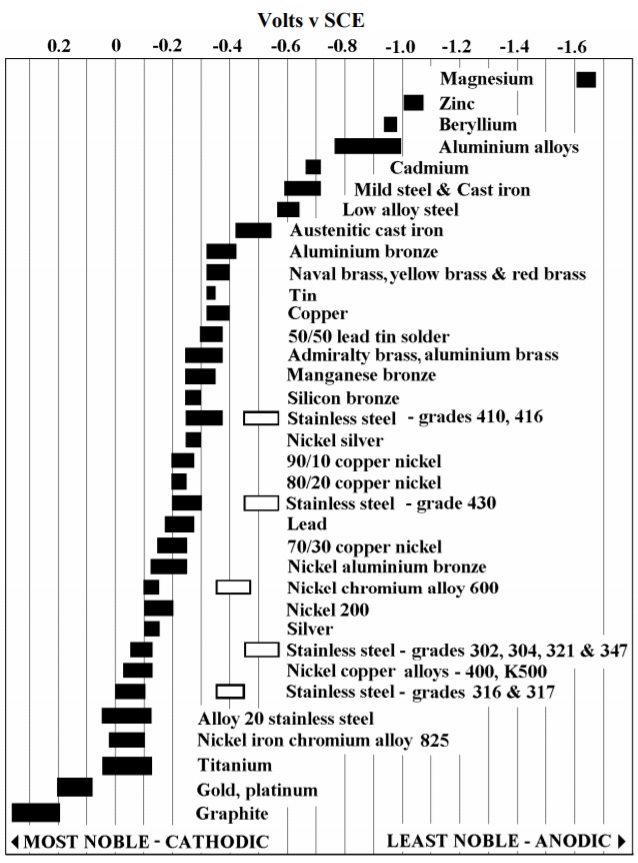
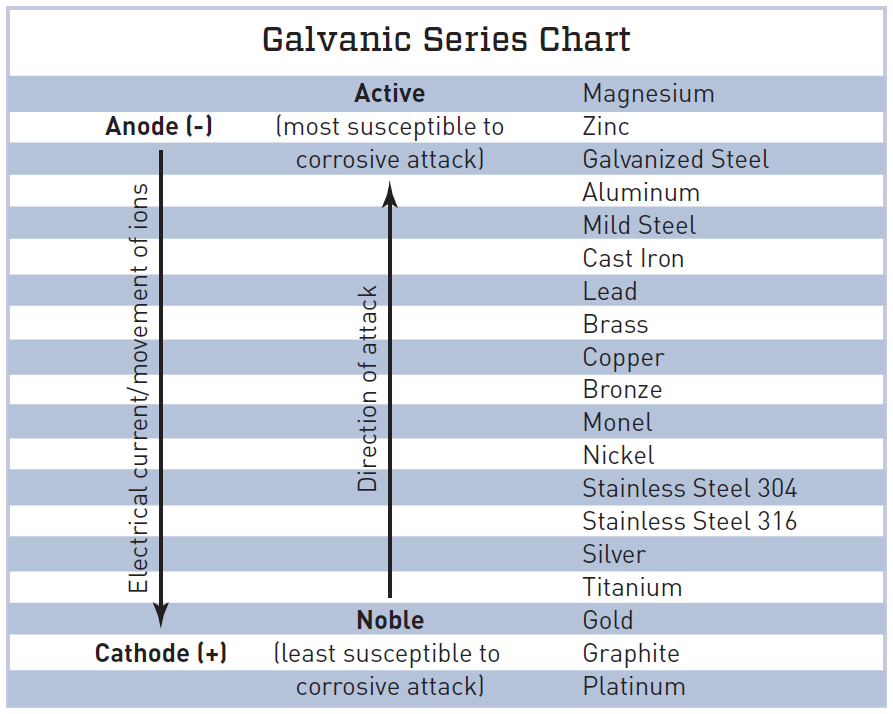
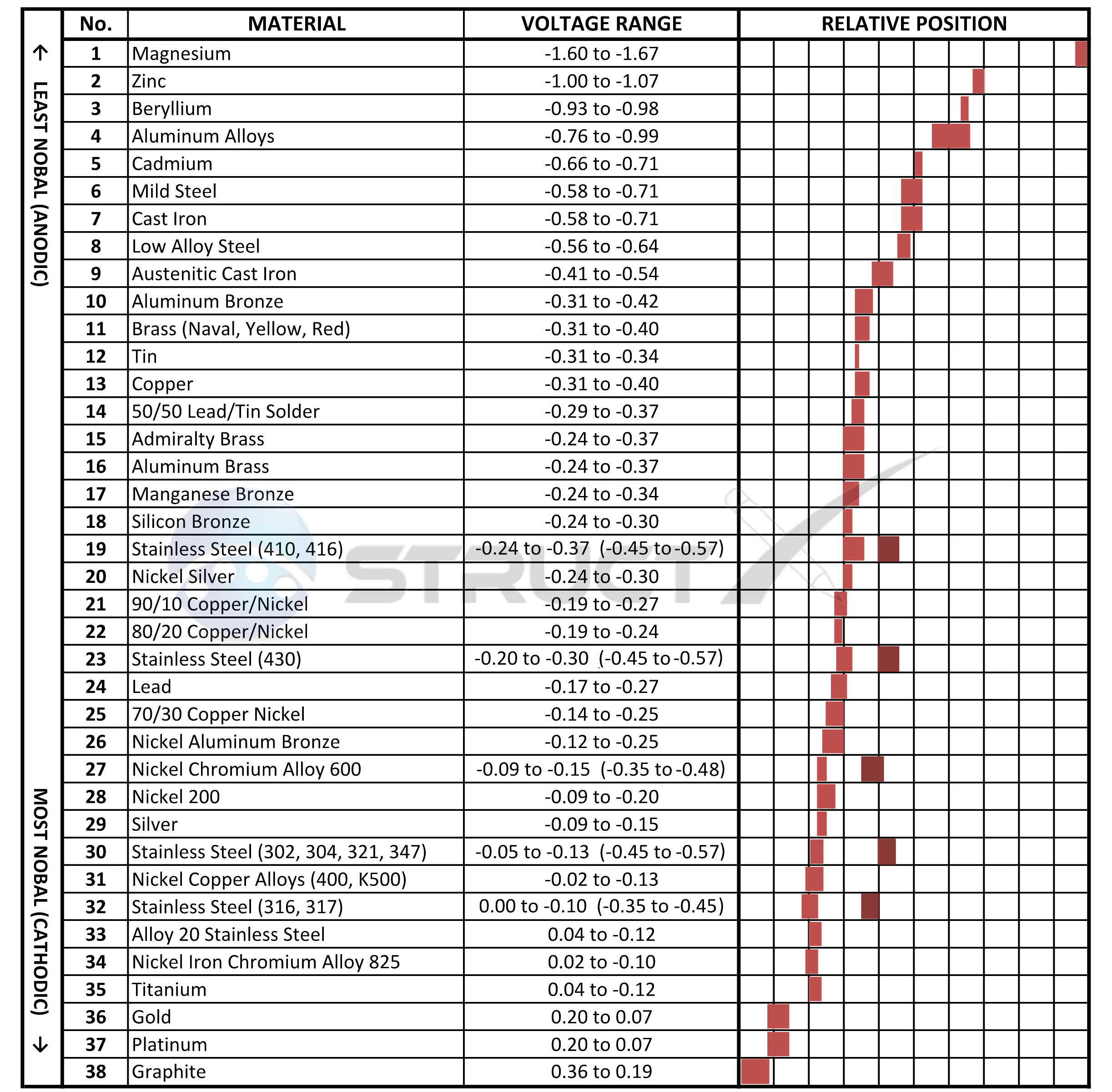
Galvanic Chart For Metals - Below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Electric current flows from plus to minus. Web view this chart of galvanic compatibility. Web typically, the presence of an electrolyte (eg. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Zn, ag/agcl and cu/cuso4 reference electrodes galvanic series chart. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. The list begins with the more active (anodic) metal and proceeds down. Water) is necessary to promote galvanic corrosion. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web all metals can be classified into a galvanic series representing the electrical potential they develop in a given electrolyte against a standard reference electrode. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal. Web what is galvanic corrosion: This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion. Use this chart below to better. Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection of metals having minimal tendency to interact galvanically, or will indicate the need or degree of protection to be applied to lessen the expected potential interactions.in general, the further apart the materials are in the galvanic. The relative position of two metals. Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Align the metal to be assessed for the risk of corrosion in the rows (coordinating metal) with the contact metal. In this article, we will discuss what is galvanic corrosion, its applications, and how to. Maximum recommended voltage difference is 0,2v: Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: First there must be two electrochemically dissimilar metals present. Aluminum 1100, 3003, 3004, 5052, 6053. The list begins with the more active (anodic) metal and proceeds down. Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Web all metals can be classified into a galvanic series representing the electrical potential they develop in a given electrolyte against a standard reference electrode. First there must be two electrochemically dissimilar metals present. Web. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web all metals can be classified into a galvanic series representing the electrical potential they develop in a given electrolyte against a standard reference electrode. Use this chart below to better understand what metals will work best together without potential for. The relative position of two metals on such a series gives a good indication of. Water) is necessary to promote galvanic corrosion. Web below is a galvanic reaction chart for dissimilar metals. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: Aluminum 1100, 3003, 3004, 5052, 6053. Web the table below is the galvanic series of metals, alloys and graphite in seawater (most noble at top) in flowing seawater, at ‘normal’ temperature. Though the order of metals in a galvanic series remains the same in most conducting solutions, some. Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection. These fi lms give them exceptionally good corrosion resistance, although they are among the most active metals. First there must be two electrochemically dissimilar metals present. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web typically, the presence of. The chart can be used to determine the likelihood of a galvanic reaction, and galvanic corrosion or bimetallic corrosion, between two different metals in a seawater environment. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. Web what is galvanic corrosion: There are three conditions that must exist for galvanic corrosion to occur. When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion. Align the metal to be assessed for the risk of corrosion in the rows (coordinating metal) with the contact metal. Web the galvanic series of metals (right) lists metals and alloys in decreasing order of electrical activity. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Zn, ag/agcl and cu/cuso4 reference electrodes galvanic series chart. Web fastener material selection based on the galvanic series of metals to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. Web typically, the presence of an electrolyte (eg. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Metals nearer the top of the table are less noble metals and have a greater tendency to lose electrons than the more noble metals found lower on the list. Water) is necessary to promote galvanic corrosion. Though the order of metals in a galvanic series remains the same in most conducting solutions, some. Use this chart below to better understand what metals will work best together without potential for galvanic corrosion: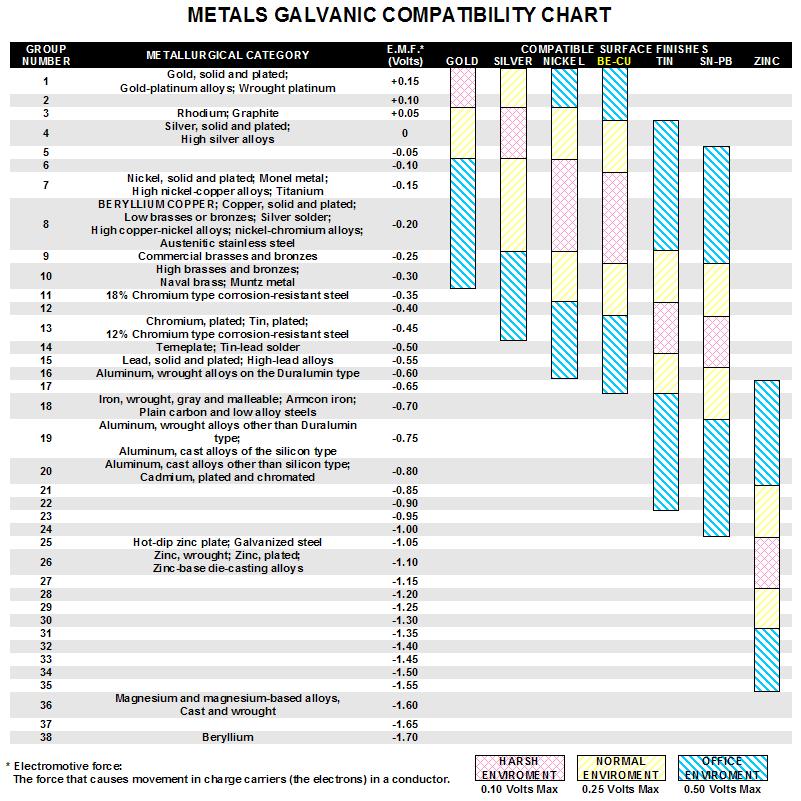
Omega Shielding Products Metals Galvanic Compatibility Chart
An Introduction to the Galvanic Series Galvanic Compatibility and

Metals Galvanic Compatibility Chart Online Shopping
Separating Galvanic Metals JLC Online

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot

The Galvanic Series the essential guide EngineeringClicks

Galvanic Chart Of Metals
![Galvanic Corrosion [with Chart] EngineerExcel](https://engineerexcel.com/wp-content/uploads/2023/03/galvanic-corrosion-chart.png)
Galvanic Corrosion [with Chart] EngineerExcel

Galvanic Chart Of Metals
Galvanic Series (electrochemical series)
The Relative Position Of Two Metals On Such A Series Gives A Good Indication Of.
Web This Chart Will Help You To Determine Which Metals Are More Noble Than Other Metals.
Web The Galvanic Series Chart Below Shows Metals And Their Electrochemical Voltage Range (Relative Activity In Flowing Sea Water).
First There Must Be Two Electrochemically Dissimilar Metals Present.
Related Post: