How Do You Draw A Water Molecule
How Do You Draw A Water Molecule - Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. The differences in electronegativity and lone electrons give oxygen a partial negative charge and each hydrogen a partial positive charge. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar. Chemists normally represent a bond using a line instead of two dots. Web from there, we will determine the structural formula of water. The structures of h 2 , f 2 , and h 2 o would usually be drawn as follows: How do they organize themselves in the three states (liquid, solid, gas)? As we can see, the 2 hydrogen atoms are covalently bonded to the oxygen atom, which has two lone pairs (4 total electrons that push the h atoms further away). To be the center atom, ability of having greater valance is important. First off, looking at the chemical formula for h2o we could draw something like this to describe its structure. Water is an excellent solvent. New data show that the virus can hop back and forth between cows and birds, a trait that could allow it to spread across wide. Students will be able to explain, on the molecular level, what. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. This is because oxygen is more electronegative, meaning that it is better than hydrogen at attracting electrons. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o molecule. Water is an excellent solvent.. To be the center atom, ability of having greater valance is important. There are two lone pairs of electron. Web sara reardon is a freelance journalist based in bozeman, montana. Web a water molecule is made up of two hydrogen atoms and one oxygen atom. Web one of the most common examples is the water molecule, made up of one. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. Web water is a bent molecule because of the two lone pairs on the central oxygen atom. The differences in electronegativity and lone electrons give oxygen a partial negative charge and each hydrogen a partial positive charge. Web in this video we. Water is an excellent solvent. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar. The differences in electronegativity and lone electrons give oxygen a partial negative charge and each hydrogen a partial positive charge. Web from there, we will determine the structural formula of water. The individual dipoles point from the. Web water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure. Web there are a few ways we can think about the molecular structure of h2o. Web a water molecule is made up of two hydrogen atoms and one oxygen atom. As we can see, the 2. Web one of the most common examples is the water molecule, made up of one oxygen atom and two hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Center atom of h 2 o. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Web there are a few ways we can think about the molecular structure of h2o. Center atom of h 2 o. New data show that the virus can hop back and forth between cows and birds, a trait that could allow. It is one of the easiest atoms to build a model of, and is therefore an excellent starting point for students learning to build molecular models. Web one of the most common examples is the water molecule, made up of one oxygen atom and two hydrogen atoms. How do they organize themselves in the three states (liquid, solid, gas)? Web. We cover how and why is water a solvent to other substances, and how the ability of water to act as a solvent makes it a great transporting. First off, looking at the chemical formula for h2o we could draw something like this to describe its structure. Explain what is meant by hydrogen bonding and the molecular structural features that. The structures of h 2 , f 2 , and h 2 o would usually be drawn as follows: It is a simple molecule, consisting of just one oxygen atom and two hydrogen atoms. How do they organize themselves in the three states (liquid, solid, gas)? Because of the shape, the dipoles do not cancel each other out and the water molecule is polar. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Students will be able to explain, on the molecular level, what makes water a polar molecule. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. When two hydrogen atoms are bound to an oxygen atom, the outer electron shell of oxygen is filled. A single oxygen atom contains six electrons in its outer shell, which can hold a total of eight electrons. Students will be able to explain, on the molecular level, what makes water a polar molecule. Web one of the most common examples is the water molecule, made up of one oxygen atom and two hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web in this video we discuss the structure of water. The differences in electronegativity and lone electrons give oxygen a partial negative charge and each hydrogen a partial positive charge. Web sara reardon is a freelance journalist based in bozeman, montana. Total electron pairs are determined by dividing the number total valence electrons by two.
The Structure and Properties of Water / Introduction to Chemistry
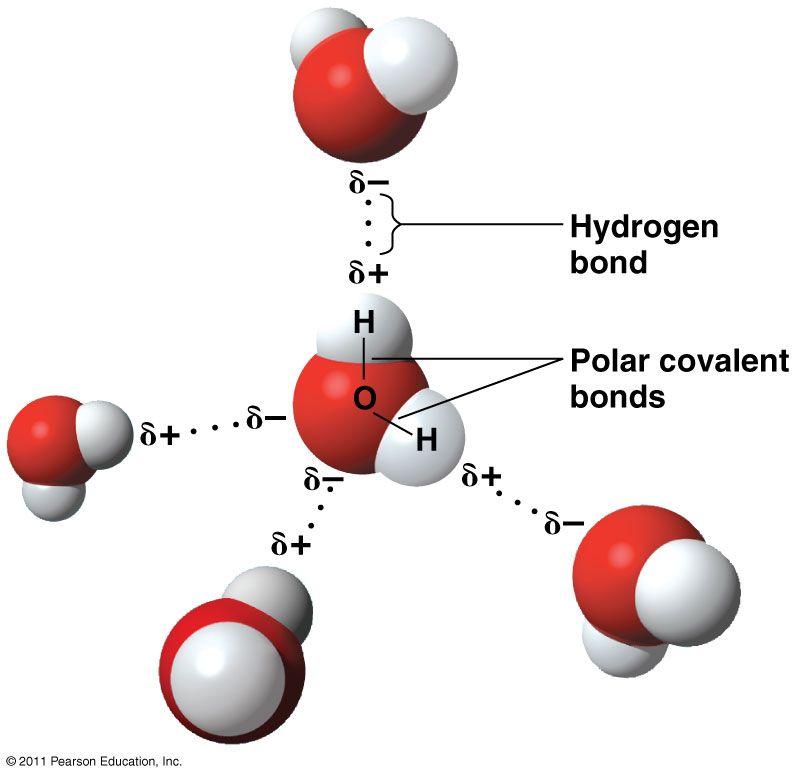
Diagram Of Water Molecule Labeled

Diagram Of Water Molecule
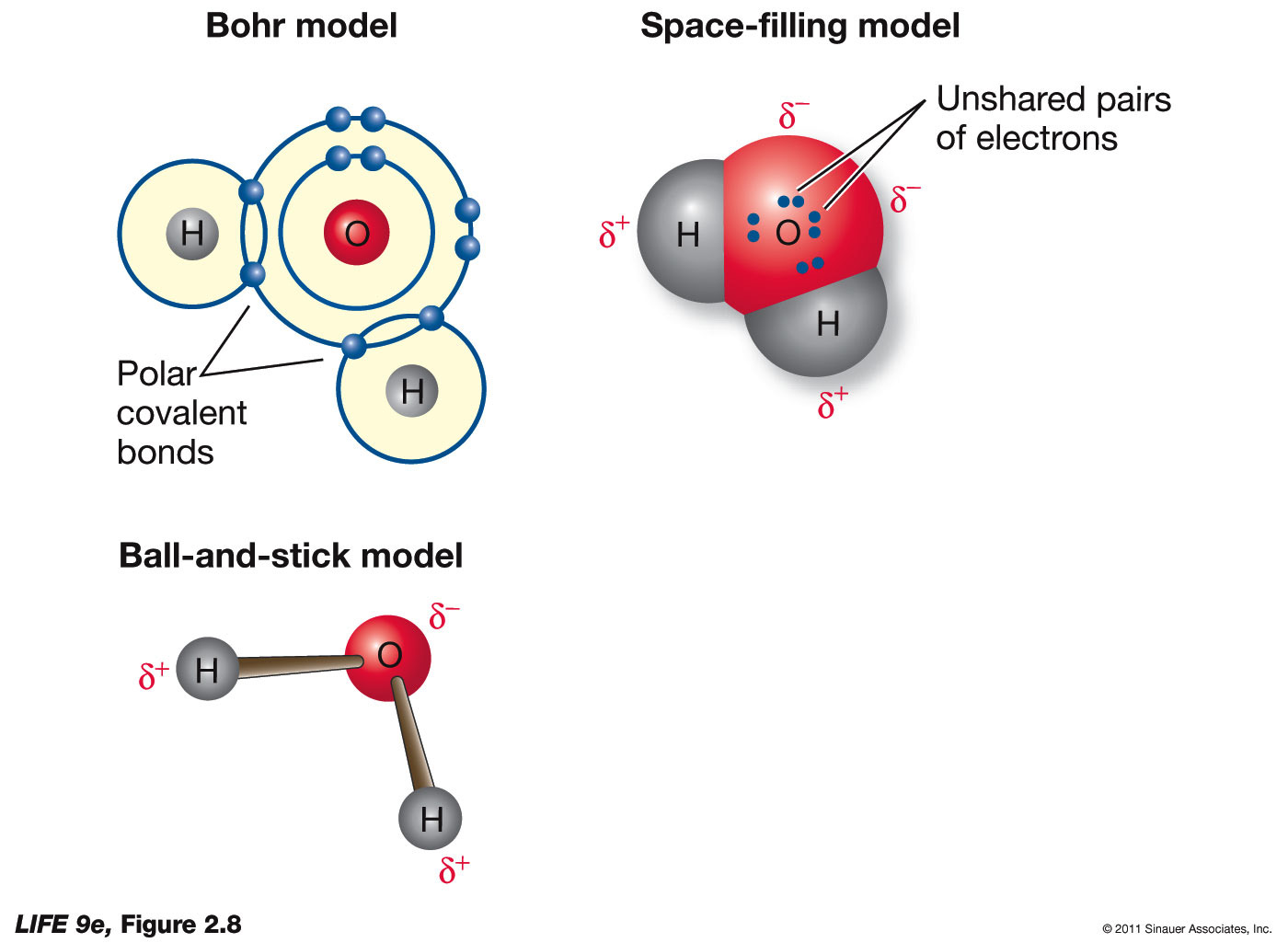
The BioLogs CAPE 1 Water Introduction to it's BIOchemistry
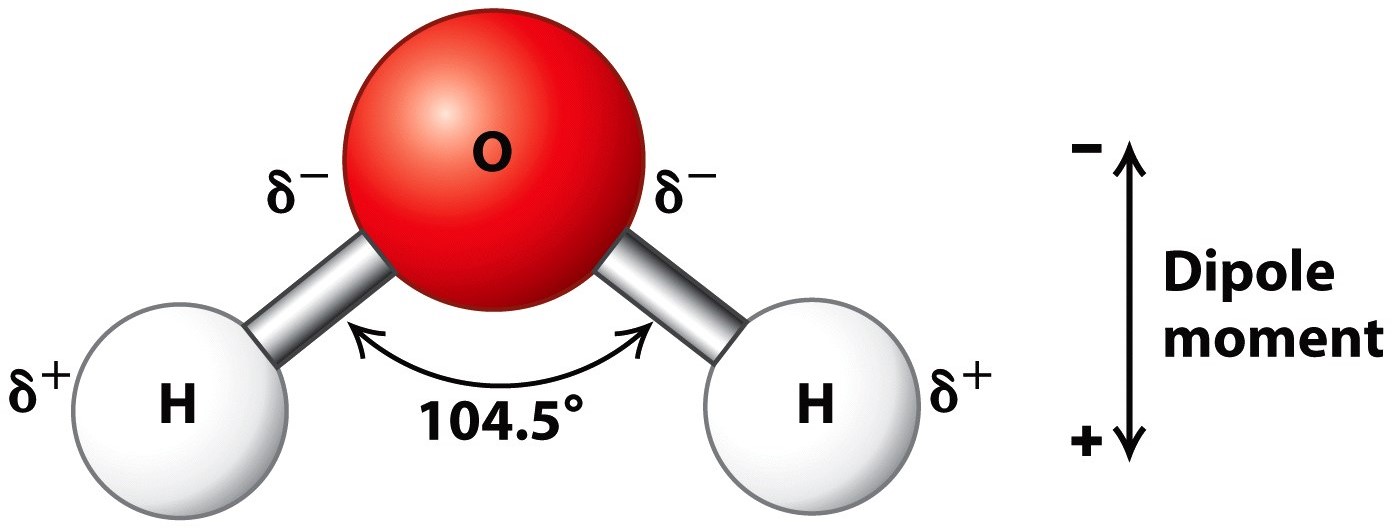
Describe the Structure of a Water Molecule

Diagram Of Water Molecule
Diagram Of Water Molecule Labeled vrogue.co
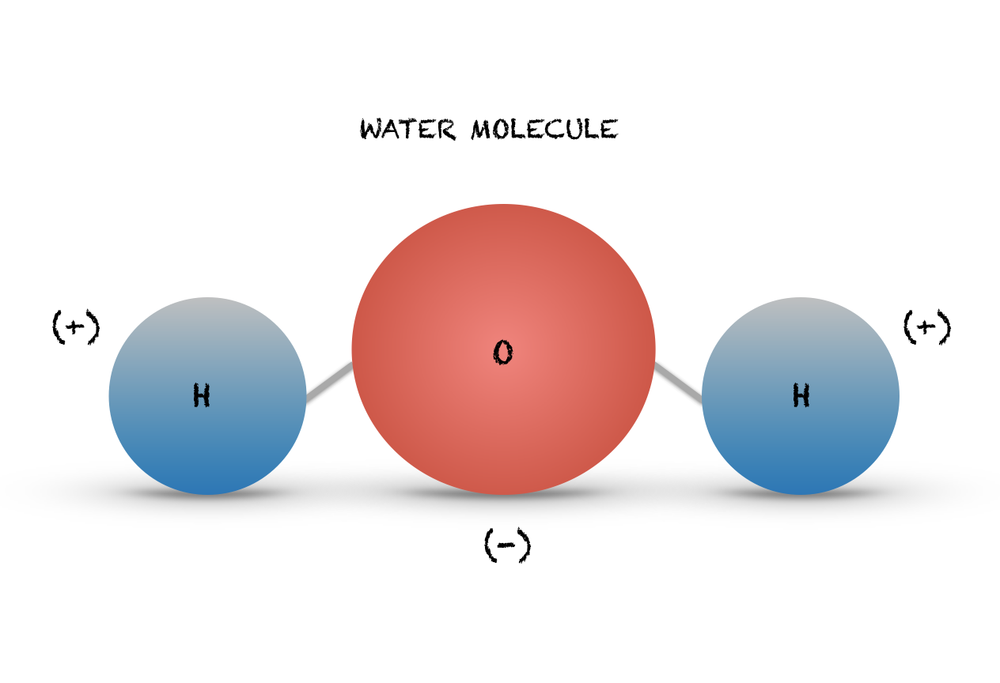
32 Draw And Label A Water Molecule Labels 2021

Diagram Of Water Molecule
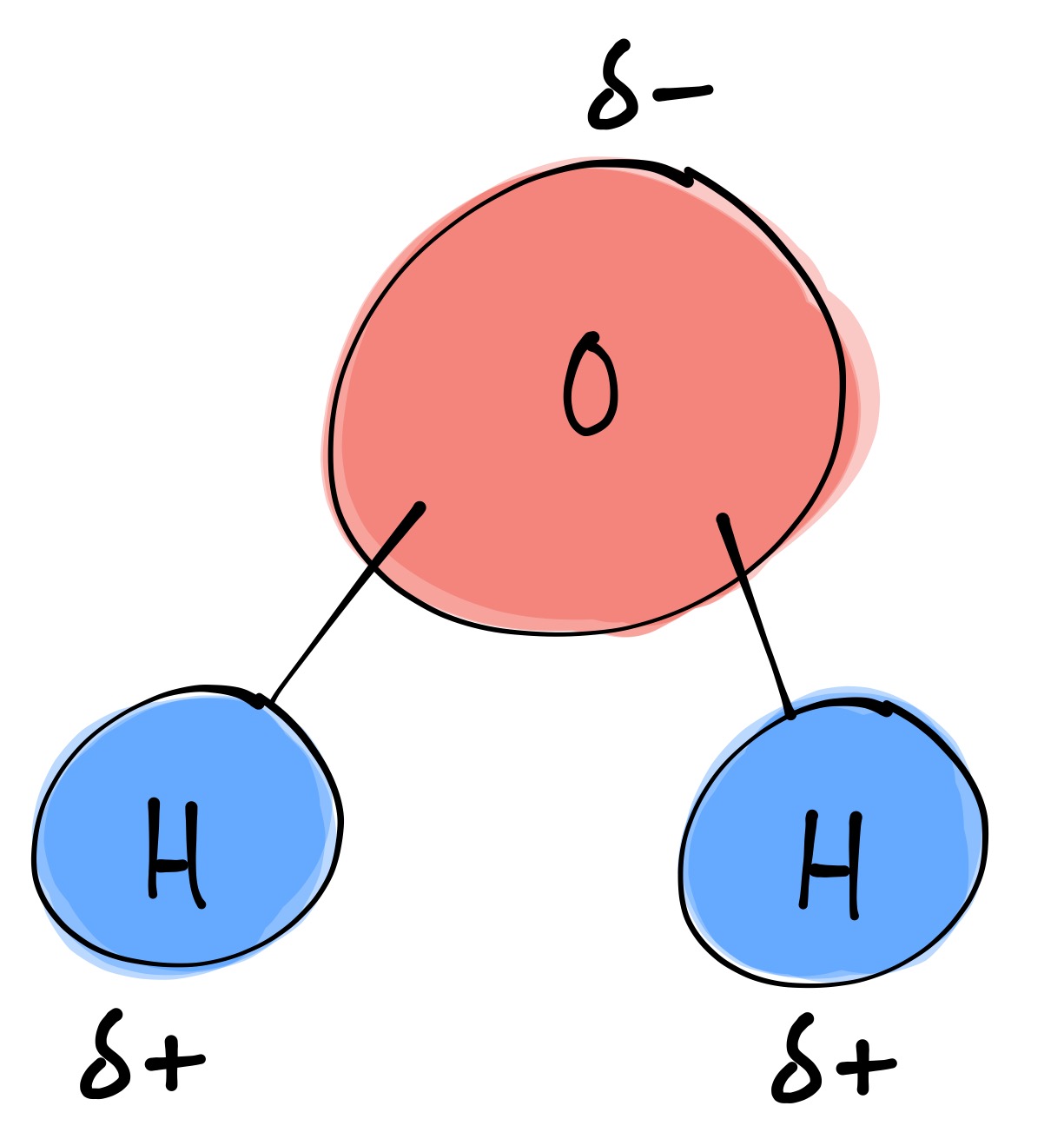
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule
This Is Because Oxygen Is More Electronegative, Meaning That It Is Better Than Hydrogen At Attracting Electrons.
To Be The Center Atom, Ability Of Having Greater Valance Is Important.
This Is Because The Oxygen Atom, In Addition To Forming Bonds With The Hydrogen Atoms, Also Carries Two Pairs Of Unshared Electrons.
For, H 2 O, Total Pairs Of Electrons Are 4 In Their Valence Shells.
Related Post: