How To Draw H2O
How To Draw H2O - Calculate the total number of valence electrons in the molecule. For h2o, this would be 2 + 6 = 8, since hydrogen has 1 valence electron and oxygen has 6. Each bond consists of a pair of electrons. If you’ve ever wondered what the h2o electron dot diagram looks like, you’ve come to the right place. #3 if needed, mention formal charges on the atoms. Determine the total number of valence electrons. Web drawing the lewis structure for water. Because water molecule is simple, some of these steps are not used much. There are two lone pairs of electrons on each oxygen atom (represented by. We draw lewis structures to predict: Web the lewis structure of h2o, or water, can be drawn by following these steps: O has 6 valence electrons, and each h has one. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. #1 draw a rough skeleton structure. There are two lone pairs. Oxygen is a group via element with six electrons in. In such cases, they are mentioned with respective steps. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. Web lewis structure of h2o (or water) contains single bonds between the oxygen (o) atom and each hydrogen (h) atom. For the h2o structure use the. With the lewis structure for water (h 2 o) remember that water only needs two valence electrons to have a full outer shell. It should look like this with the single line representing the sharing of two electrons between them. O has 6 valence electrons, and each h has one. We draw lewis structures to predict: #1 draw a rough. The first step in drawing the h2o lewis structure is to count the valence electrons. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. O has 6 valence electrons, and each h has one. The oxygen atom have 2 lone pairs. Web drawing the lewis structure for water. Web lewis structure of h2o (or water) contains single bonds between the oxygen (o) atom and each hydrogen (h) atom. #1 draw a rough skeleton structure. Make sure you put the correct atom at the center of the water (h 2 o) molecule. Web drawing the lewis structure for water. Web i quickly take you through how to draw the. An electron dot diagram is a visual representation of the. Web you can find a procedure for drawing lewis structures at this location. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o molecule. Write the correct skeletal structure for the molecule. For h 2 o molecule, its lewis structure and. An electron dot diagram is a visual representation of the. Web draw a single bond between the oxygen atom and each hydrogen atom to represent the sharing of electrons. Web the lewis structure of h2o, or water, can be drawn by following these steps: Let’s draw and understand this lewis dot structure step by step. Because water molecule is simple,. #3 if needed, mention formal charges on the atoms. I also go over hybridization, shape and bond angle. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. With the lewis structure for water (h 2 o) remember that water only needs two valence electrons to have a full outer shell.. Web how to draw electron dot structure for waterelectron dot structurelewis dot structurehow to draw electron dot structurehow to draw lewis dot structure for h2. Web first, we need to draw the lewis structure of h 2 o. First, determine the total number of valence. In short, these are the steps you need to follow for drawing a lewis structure:. Web how to draw an h2o electron dot diagram: For h2o, this would be 2 + 6 = 8, since hydrogen has 1 valence electron and oxygen has 6. I also go over hybridization, shape and bond angle. The geometrical structure of the h2o molecule. For the h2o structure use the periodic table to find the total number of valence. Let’s draw and understand this lewis dot structure step by step. For h 2 o molecule, its lewis structure and those steps are explained in detail in this tutorial. For h2o, this would be 2 + 6 = 8, since hydrogen has 1 valence electron and oxygen has 6. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Place the least electronegative atom hydrogen in the centre of the structure. In short, these are the steps you need to follow for drawing a lewis structure: With the lewis structure for water (h 2 o) remember that water only needs two valence electrons to have a full outer shell. #2 mention lone pairs on the atoms. For the h2o structure use the periodic table to find the total number of valence electrons for.more. Web first, we need to draw the lewis structure of h 2 o. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. It should look like this with the single line representing the sharing of two electrons between them. The first step in drawing the lewis structure of water is to determine the total number of. The oxygen atom (o) is at the center and it is surrounded by 2 hydrogen atoms (h). Web i quickly take you through how to draw the lewis structure of water, h2o. The first step in drawing the h2o lewis structure is to count the valence electrons.
HOW TO DRAW WATER Step by Step Drawing Tutorial easiest way to draw

Water Drawing How To Draw Water Step By Step

How To Draw Water Step By Step
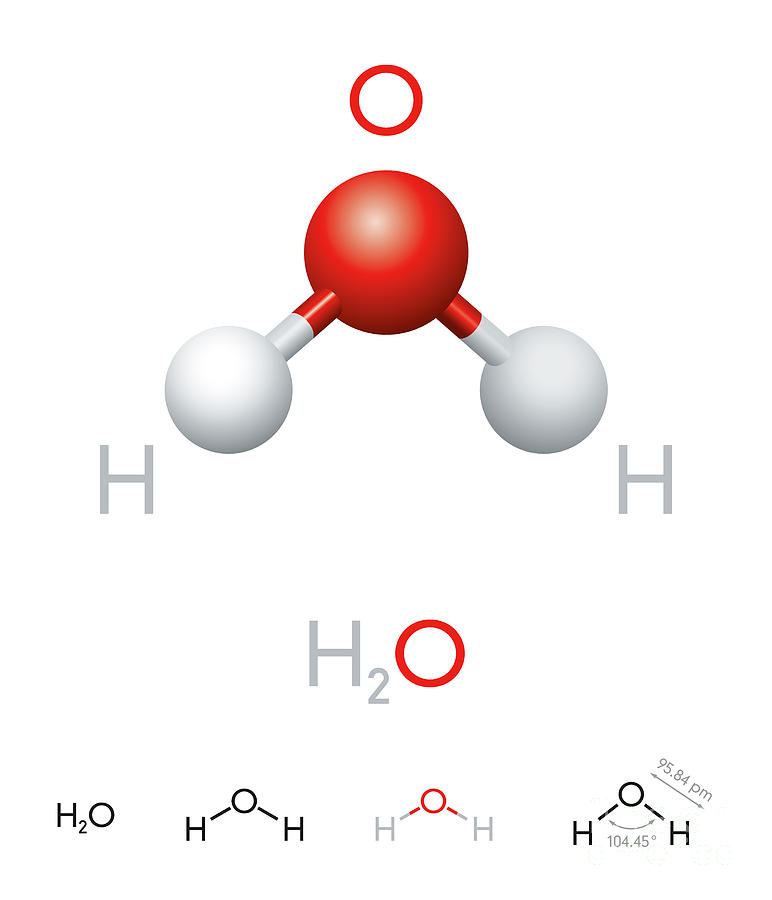
H2O Water molecule model and chemical formula Digital Art by Peter

How to Draw Water JovanytinRiddle
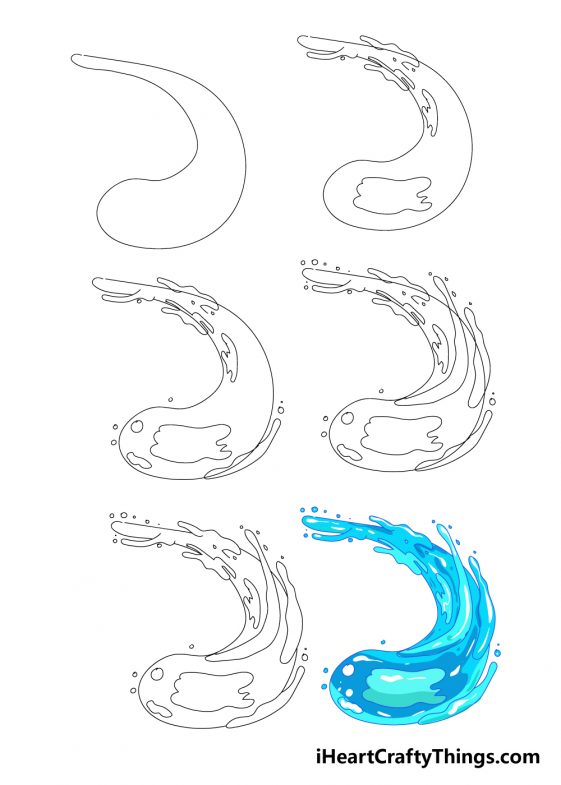
Water Drawing How To Draw Water Step By Step

How to draw Water by Sessp on DeviantArt Digital Painting Techniques

H2o water molecule model chemical formula Vector Image

Water Lewis Structure How to Draw the Lewis Structure for Water YouTube

How to Draw Water Step by Step Guide How to Draw
Each Bond Consists Of A Pair Of Electrons.
For H₂O, O Must Be The Central Atom.
Just Add Water Logo On Your Computer Using Microsoft Paint.
Π Mo Diagram For Nitrate Ion.
Related Post: