Isotope Drawing
Isotope Drawing - Refer to the periodic table and use the number of protons to identify the element. Visualize trends, 3d orbitals, isotopes, and mix compounds. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. Web an element with three stable isotopes has 82 protons. Deuterium is an isotope of hydrogen proton. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web have students draw different isotopes of the same element with pens or markers. Web this online quiz is intended to give you extra practice in writing names and notation for hundreds of elements and isotopes. Web we recommend using the latest version of chrome, firefox, safari, or edge. Web how to draw the atomic structure of atoms. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei. Manipulate the atomic weight equation to calculate various unknown variables. Identify the element and write symbols for the isotopes. Web learn and interactive with isotopes. Web how to draw the atomic structure of atoms. Web draw a bohr model of the isotope on the paper ; Distinguish between atomic weight, atomic number, and mass number. Learn how to identify different isotopes of an element, how to calculate the average atomic mass from isotopic abundance, and how to compare the properties of isotopes. The relative abundance of an isotope is the percentage of atoms with. Web how to draw the atomic structure of atoms. Manipulate the atomic weight equation to calculate various unknown variables. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Every chemical element has one or more isotopes. Web learn and interactive with isotopes. The number of protons and the mass number of an atom define the type of atom. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Made with by wei zheng joe taylor. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei. Web explore the concept of. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web interactive periodic table showing names, electrons, and oxidation states. If you understand how protons and electrons relate to one another, as well as how neutrons aid in. Web have students draw different isotopes of the same element with pens or markers. Why is isotope notation important? Web draw a bohr model of the isotope on the paper ; Are stable, occurring in a natural proportion of approximately 93:1. There are three naturally occurring isotopes of carbon: Web have students draw different isotopes of the same element with pens or markers. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web isotope, one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical. Every chemical element has one or more isotopes. All atoms of the same element have the same number of protons, which is the atomic number of that element. Calculate atomic weight from percent abundance. Then play a game to test your ideas! Web this online quiz is intended to give you extra practice in writing names and notation for hundreds. Are stable, occurring in a natural proportion of approximately 93:1. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Web draw a bohr model of the isotope on the paper ; Refer to the periodic table and use the number of protons to identify the element. Web the isotopes concept builder challenges. Deuterium is an isotope of hydrogen proton. The first activity consists of all multiple choice questions that target a basic understanding of fundamental. Web isotope, one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical. Then play a game to test your ideas! The atomic number of carbon is 6, which means. Learn how to identify different isotopes of an element, how to calculate the average atomic mass from isotopic abundance, and how to compare the properties of isotopes. It can also plot either calculated or manually inputted isotopic distributions. Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei. This program can calculate the isotopic distribution of a given chemical species. Use the periodic table and bohr model to identify the name of the isotope If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. The first activity consists of all multiple choice questions that target a basic understanding of fundamental. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Use red ink for protons and black ink for electrons. These standards provide guidelines to assist the council in applying those criteria in iowa code sections 135.64(1) a to r and 135.64(3). Web we recommend using the latest version of chrome, firefox, safari, or edge. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Refer to the periodic table and use the number of protons to identify the element. Web the number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.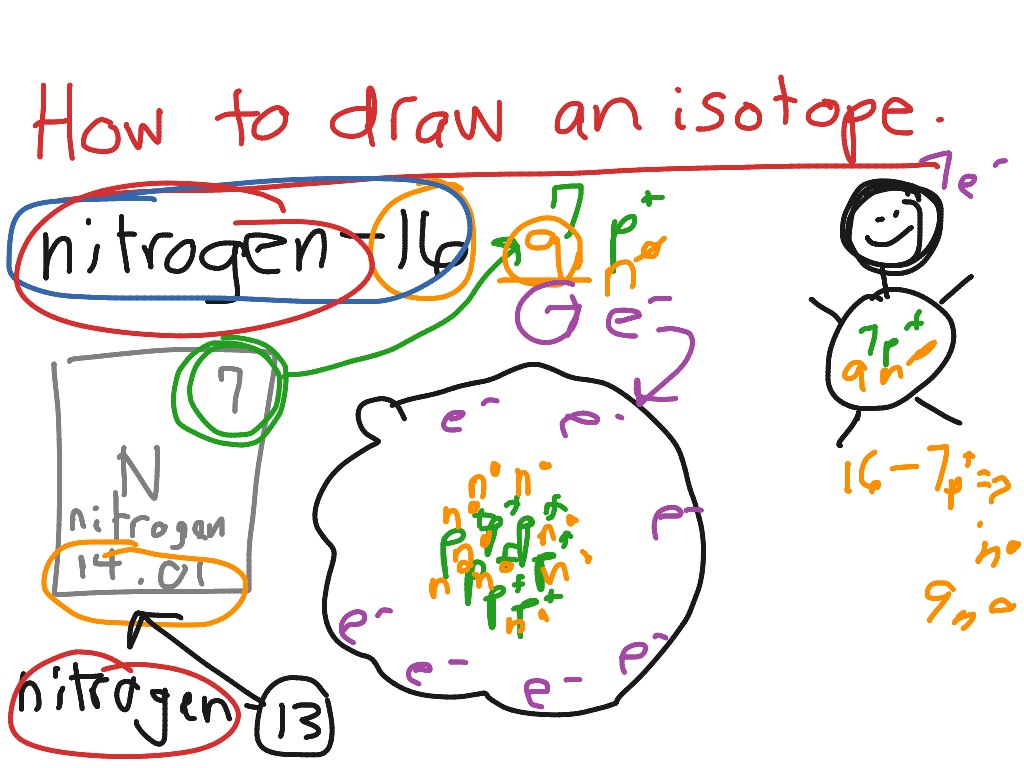
How to Draw an Isotope Science ShowMe

Isotopes of carbon, illustration Stock Image C028/6464 Science
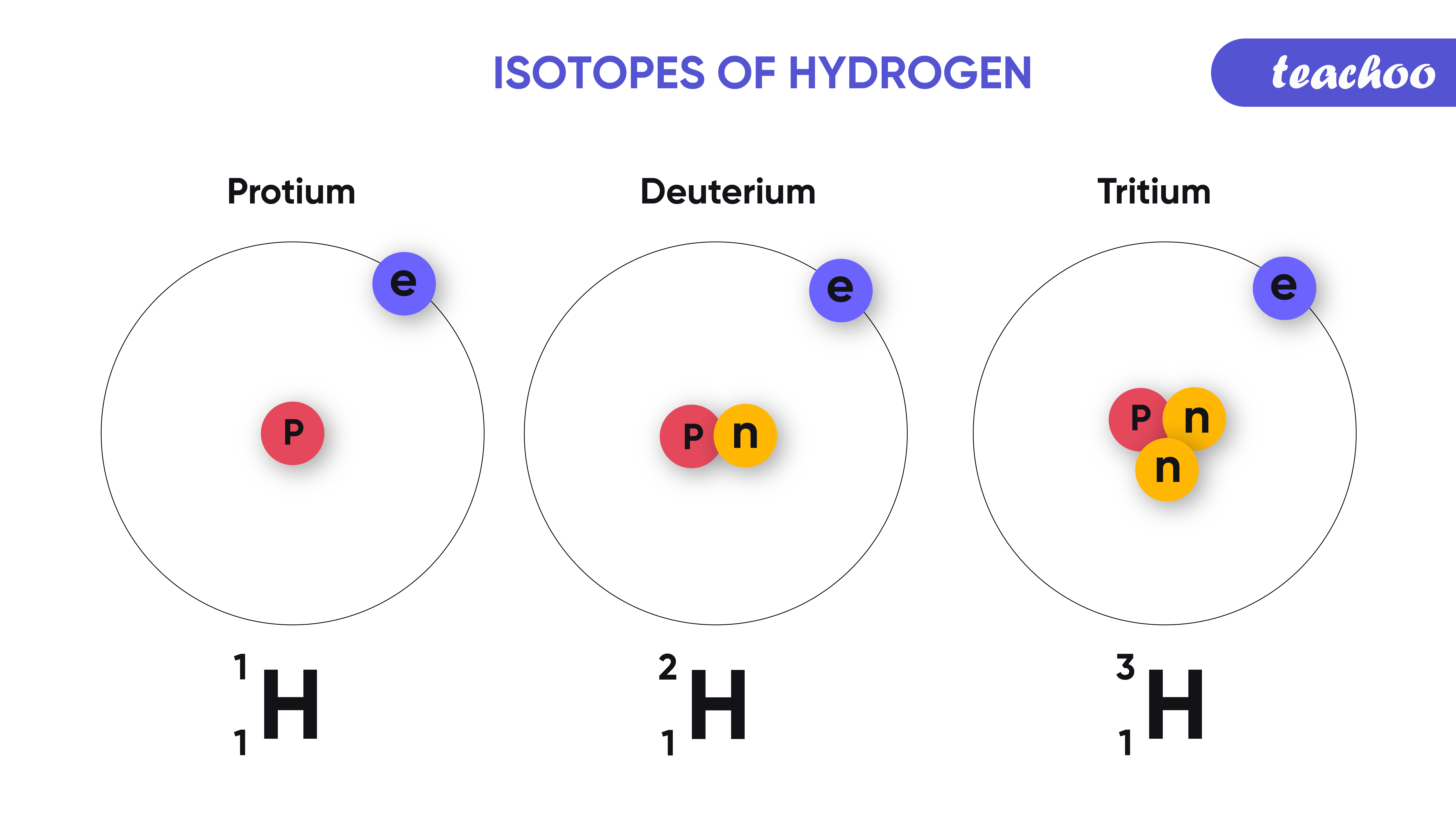
Isotopes and Isobars Definition, Uses and Difference Teachoo
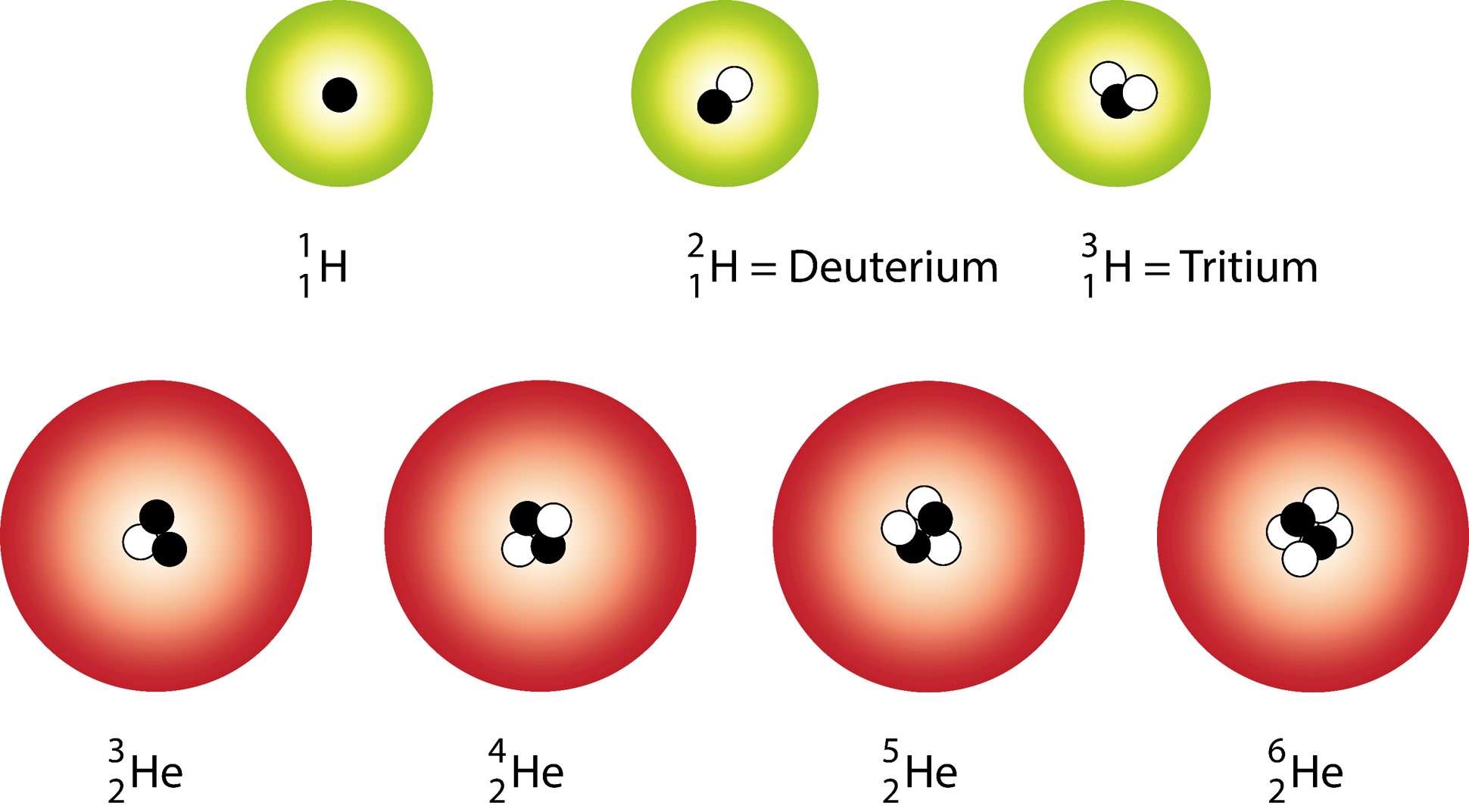
PreChemistry
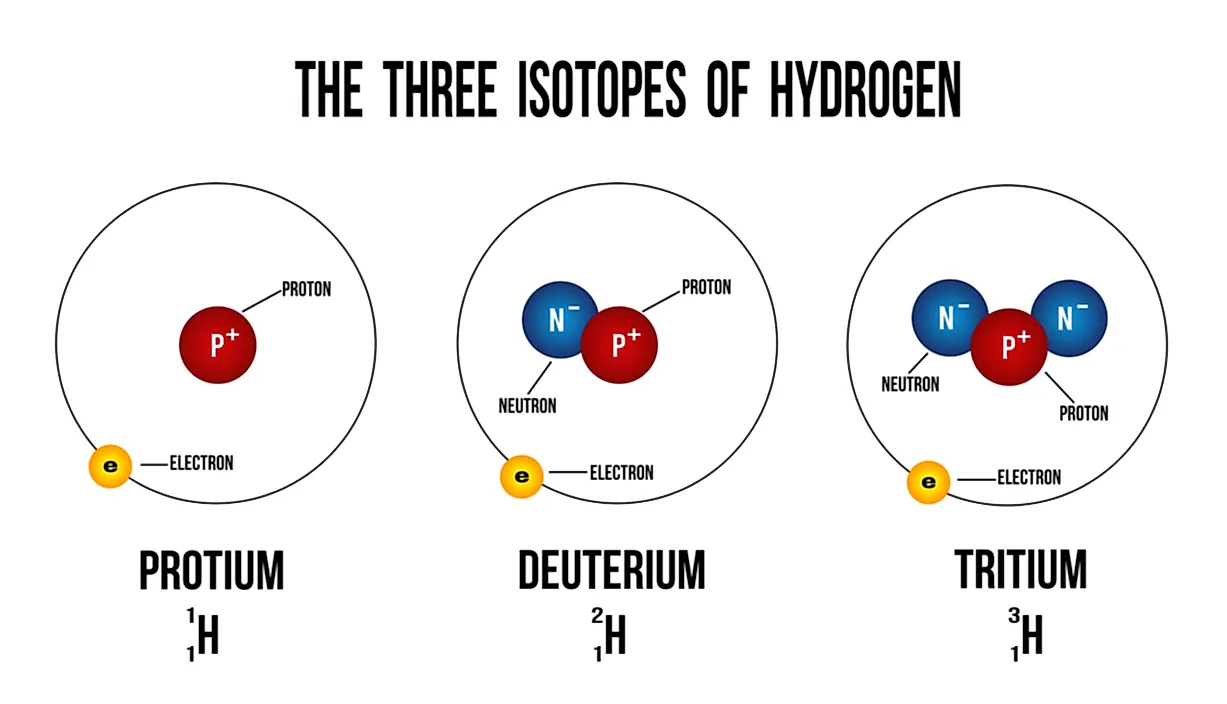
What Is an Isotope? WorldAtlas
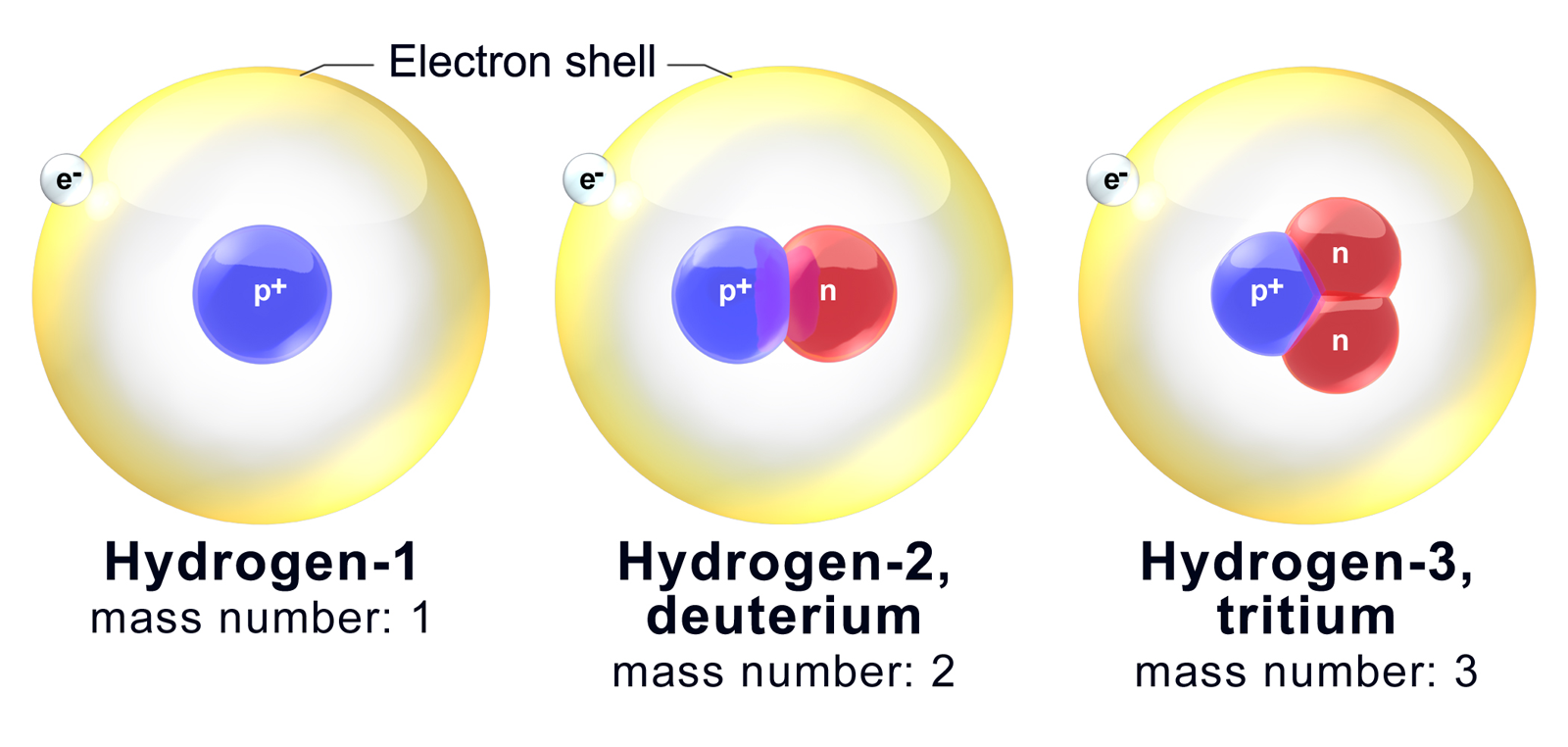
DOE Explains...Isotopes Department of Energy
How to Calculate Isotopes Sciencing
/Isotope-58dd6b415f9b5846830254ae.jpg)
Isotopes Definition and Examples in Chemistry
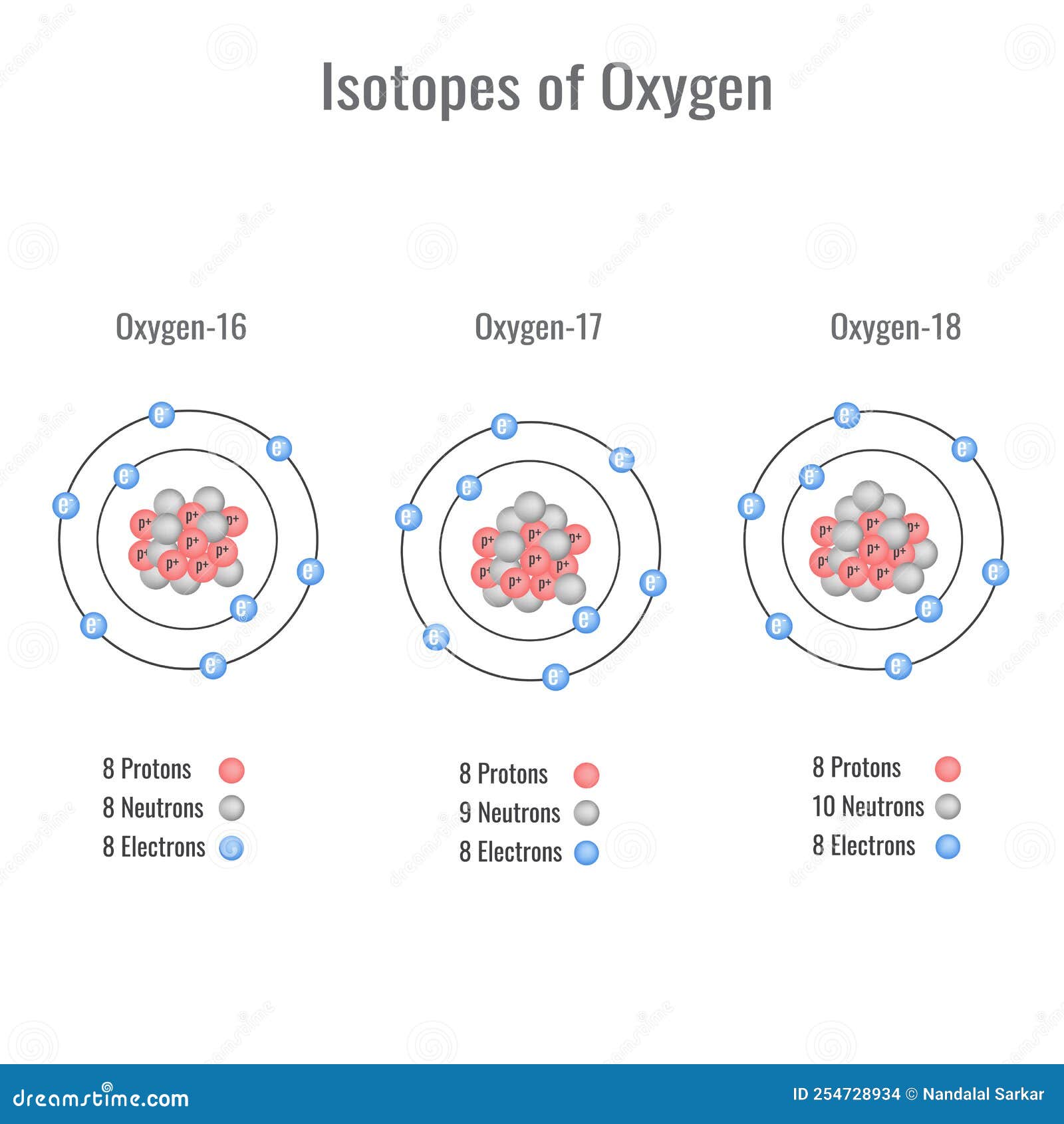
Isotopes of Oxygen Vector Illustration Stock Vector Illustration of

Isotopes What are Isotopes? Relative Atomic Mass
Distinguish Between Atomic Weight, Atomic Number, And Mass Number.
Build An Atom Out Of Protons, Neutrons, And Electrons, And See How The Element, Charge, And Mass Change.
There Are Three Scaffolded Activities.
Web This Online Quiz Is Intended To Give You Extra Practice In Writing Names And Notation For Hundreds Of Elements And Isotopes.
Related Post:
