Liquid Liquid Extraction Flow Chart
Liquid Liquid Extraction Flow Chart - Dissolving the mixture in the first solvent and then adding a second immiscible solvent that will selectively dissolve one. Background extraction is one of humankind’s oldest chemical operations. The extraction efficiency is modified by adjustment of ph and ionic strength in the aqueous phase. Web a very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine, dried, filtered and finally evaporated to yield a crude product or a pure product. Return the aqueous layer to the separatory funnel. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it. Of acid rco 2 h rco 2 h acid rnh 2 base n neutral extract with 5% naoh organic phase aqueous phase extract with 10% hcl evap. The preparation of a cup of coffee or tea involves the extraction of flavor and odor components from dried vegetable matter with hot water. Usually one of the solvents is water. Web solvent partitioning requires two solvents that are not miscible in each other. And (5) outgoing raffinate composition have been specified/selected. Dissolving the mixture in the first solvent and then adding a second immiscible solvent that will selectively dissolve one. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it. The extraction efficiency is modified. Of acid rco 2 h rco 2 h acid rnh 2 base n neutral extract with 5% naoh organic phase aqueous phase extract with 10% hcl evap. This article describes the process design of such systems. In its simplest form, this involves the extraction of a solute from a binary solution by bringing it into contact with a second immiscible. When you select new problem, a new phase envelope is shown and a new feed composition and feed flow rates are set. And (5) outgoing raffinate composition have been specified/selected. Top organic layer and bottom aqueous layer). The technique works well if your target compound is more soluble in. \text{ml}\) of diethyl ether (an exact amount is not necessary), as. Determining number of stages \(n\) when (1) feed rate; Background extraction is one of humankind’s oldest chemical operations. Dissolving the mixture in the first solvent and then adding a second immiscible solvent that will selectively dissolve one. Return the aqueous layer to the separatory funnel. Usually one of the solvents is water. The preparation of a cup of coffee or tea involves the extraction of flavor and odor components from dried vegetable matter with hot water. This article describes the process design of such systems. The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. Determining number of stages. Determining number of stages \(n\) when (1) feed rate; The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. Web solvent partitioning requires two solvents that are not miscible in each other. Return the aqueous layer to the separatory funnel. Determining number of stages when (1) feed. The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. Web a very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine, dried, filtered and finally evaporated to yield a crude product or a pure. The technique works well if your target compound is more soluble in. Usually one of the solvents is water. Extraction of acids or bases from neutral. Web liquid/liquid extraction is the most common technique used to separate a desired organic product from a reaction mixture or to isolate an organic substance from its natural source. Web a very typical extraction. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it. Determining number of stages when (1) feed rate; Web a very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine,. Web ethyl acetate, dichloromethane, and their mixtures are among the preferred extraction solvents for phenylureas, triazoles, amides, carbamates, benzimidazoles, and chlorotriazines. When you select new problem, a new phase envelope is shown and a new feed composition and feed flow rates are set. After these two phases have been separated, they must be purified of the solvent they contain. Background. The preparation of a cup of coffee or tea involves the extraction of flavor and odor components from dried vegetable matter with hot water. Return the aqueous layer to the separatory funnel. The technique works well if your target compound is more soluble in. Web ethyl acetate, dichloromethane, and their mixtures are among the preferred extraction solvents for phenylureas, triazoles, amides, carbamates, benzimidazoles, and chlorotriazines. The other solvent is a liquid that does not dissolve very well in water, such as diethyl ether (this is the most common type of ether, and it. When you select new problem, a new phase envelope is shown and a new feed composition and feed flow rates are set. The extraction efficiency is modified by adjustment of ph and ionic strength in the aqueous phase. Determining number of stages when (1) feed rate; Web solvent partitioning requires two solvents that are not miscible in each other. Perform a single extraction using approximately \(25 \: In its simplest form, this involves the extraction of a solute from a binary solution by bringing it into contact with a second immiscible solvent in which the solute is soluble. Background extraction is one of humankind’s oldest chemical operations. The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. After these two phases have been separated, they must be purified of the solvent they contain. The two phases are put into a device called a separatory funnel, and compounds in the system will distribute between the two phases. Top organic layer and bottom aqueous layer).
Liquid Liquid Extraction Flow Chart A Visual Reference of Charts

DNA Extraction Flow Chart

Liquid Liquid Extraction Lab Report Uitm A Flowchart Of The My XXX

Diagrammatic illustration of liquidliquid extraction (adapted from

Schematic flow diagram for the solvent extraction process. Download

Diagrammatic illustration of liquidliquid extraction (adapted from
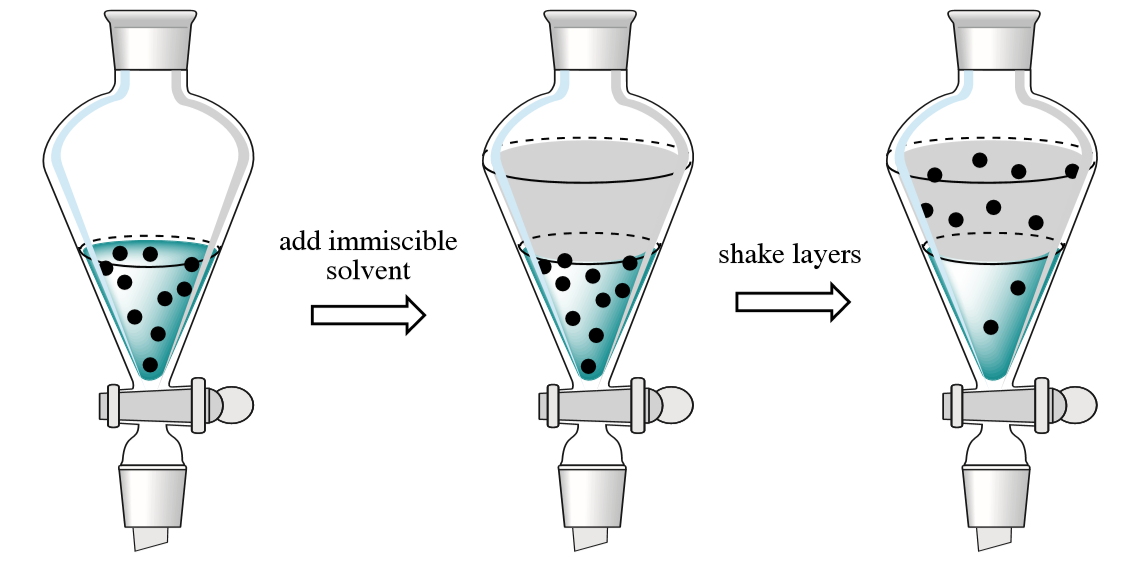
liquid liquid extraction experiment Ryan Baker
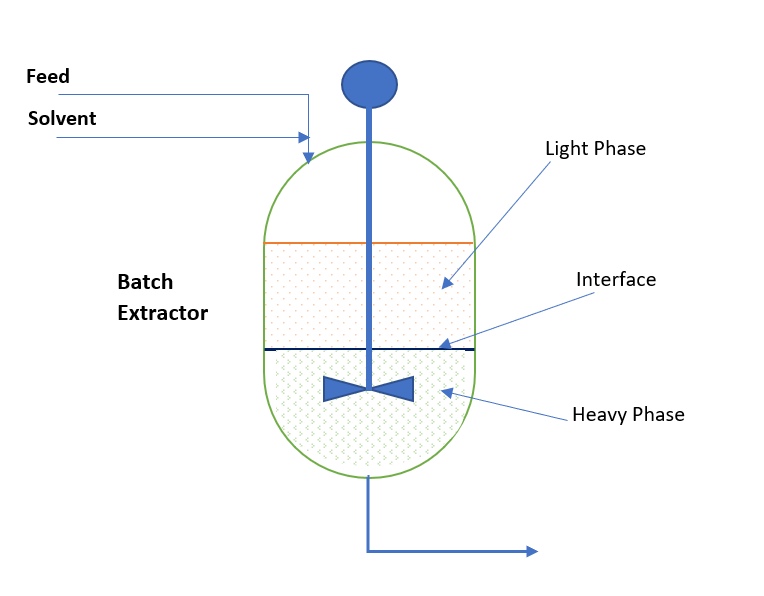
Liquid Liquid Extraction System Process Calculation ChemEnggHelp

Liquid Liquid Extraction Flow Chart
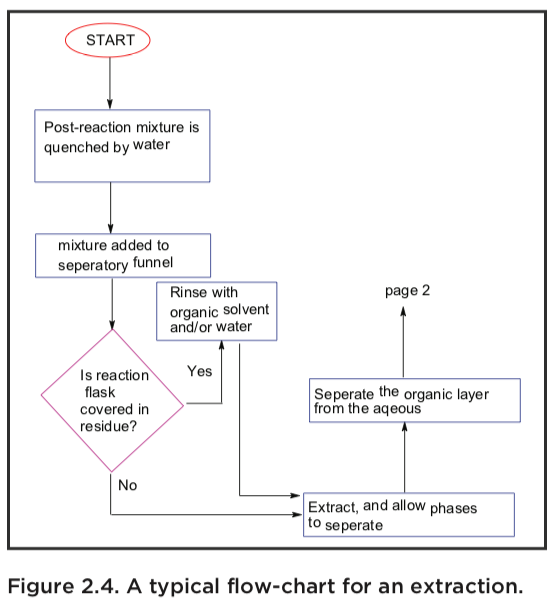
Liquid Liquid Extraction Flow Chart
Of Acid Rco 2 H Rco 2 H Acid Rnh 2 Base N Neutral Extract With 5% Naoh Organic Phase Aqueous Phase Extract With 10% Hcl Evap.
Dissolving The Mixture In The First Solvent And Then Adding A Second Immiscible Solvent That Will Selectively Dissolve One.
Web Chem 344 Extraction Flowchart.
Extraction Of Acids Or Bases From Neutral.
Related Post: