Nitrogen Gas Temperature Pressure Chart
Nitrogen Gas Temperature Pressure Chart - Please choose the units you wish to use: The molecular weight is m = 28.016 kg/kmol. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The calculation assumes that the amount and volume of gas do not change. This structure is also available as a 2d mol file or as a computed 3d sd file. Please select the species of interest : Web online calculator, figures and tables showing thermal conductivity of nitrogen, n2, at varying temperarure and pressure, si and imperial units. Web temperature is sometimes measured with a gas thermometer by observing the change in the volume of the gas as the temperature changes at constant pressure. This ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. Please be patient while the web interface loads. Web table of contents. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a. Ideal gas law equation ideal gas constant faqs. Please follow the steps below to select the data required. Web thermophysical properties of fluid systems. Please choose the units you wish to use: This structure is also available as a 2d mol file or as a computed 3d sd file. The 3d structure may be viewed using java or javascript. Please select the species of interest : Dalton's law is the final law, and it states that the combined pressure of all gases in a. Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. The 3d structure may be viewed using java or javascript. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a. Web thermophysical properties of fluid systems. Web compute the values of pressure of a gas for various temperatures. Please follow the steps below to select the data required. Web table of contents. The molecular weight is m = 28.016 kg/kmol. Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm 3 when immersed in a mixture of. What is an ideal gas? Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. The 3d structure may be viewed using java or javascript. Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. Jahangiri, m., termodynamic properties of nitrogen from the freezing line to. Web it should be noted that n« gas can be considered as an ideal gas for temperatures t > 350k and pressures p <, 10 bar. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. For real gases, the compressibility factor may be very different from one. Web the compressibility factor of an ideal gas is exactly. Web temperature is sometimes measured with a gas thermometer by observing the change in the volume of the gas as the temperature changes at constant pressure. Thermal diffusivity of nitrogen at 1, 10, 50 and 100 bara (14.5, 145, 725 and 1450 psia), and varying temperature given as °c or °f: Dalton's law is the final law, and it states. These data include the following: Web the compressibility factor of an ideal gas is exactly one. The 3d structure may be viewed using java or javascript. Accurate thermophysical properties are available for several fluids. We then have the law for an ideal gas: Web it should be noted that n« gas can be considered as an ideal gas for temperatures t > 350k and pressures p <, 10 bar. Web thermophysical properties of fluid systems. The gas constant is r = 8314.3 j/ k'kmol. What is an ideal gas? Please select the species of interest : The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm 3 when immersed in a mixture of ice and water (0.00 °c). These data include the following: Please be patient while the web interface loads. Web table of contents. Ideal gas law equation ideal gas constant faqs. Web compute the values of pressure of a gas for various temperatures using the entered temperature and the known value of pressure at that temperature. Ideal gas law equation ideal gas constant faqs. Web the compressibility factor of an ideal gas is exactly one. Please choose the units you wish to use: Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. The gas constant is r = 8314.3 j/ k'kmol. Web early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). These data include the following: Web lower limit for calculation: Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Jahangiri, m., termodynamic properties of nitrogen from the freezing line to 2000 k at pressures to 1000 mpa, j. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm 3 when immersed in a mixture of ice and water (0.00 °c). In this way table 5, rg versus p and t, can be extended beyond t = 350k. Web table of contents. Web the equations describing these laws are special cases of the ideal gas law, pv = nrt, where p is the pressure of the gas, v is its volume, n is the number of moles of the gas, t is its kelvin temperature, and r is the ideal (universal) gas constant. The calculation assumes that the amount and volume of gas do not change.
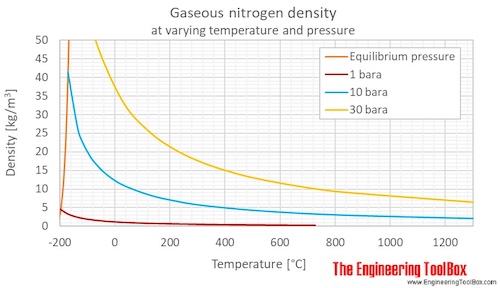
Nitrogen Density and Specific Weight vs. Temperature and Pressure

Density of nitrogen with change in temperature and at different

Nitrogen Pressure Temperature Chart A Visual Reference of Charts

Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature
Nitrogen Pressure Chart A Visual Reference of Charts Chart Master

Nitrogen Thermal Diffusivity vs. Temperature and Pressure

Nitrogen Pressure Temperature Chart

Nitrogen Thermal Conductivity vs. Temperature and Pressure

Nitrogen Thermal Diffusivity vs. Temperature and Pressure
Nitrogen Pressure Vs Temperature Chart
We Then Have The Law For An Ideal Gas:
Streng, 1971 Streng, A.g., Miscibility And Compatibility Of Some Liquid And Solidified Gases At Low Temperature, J.
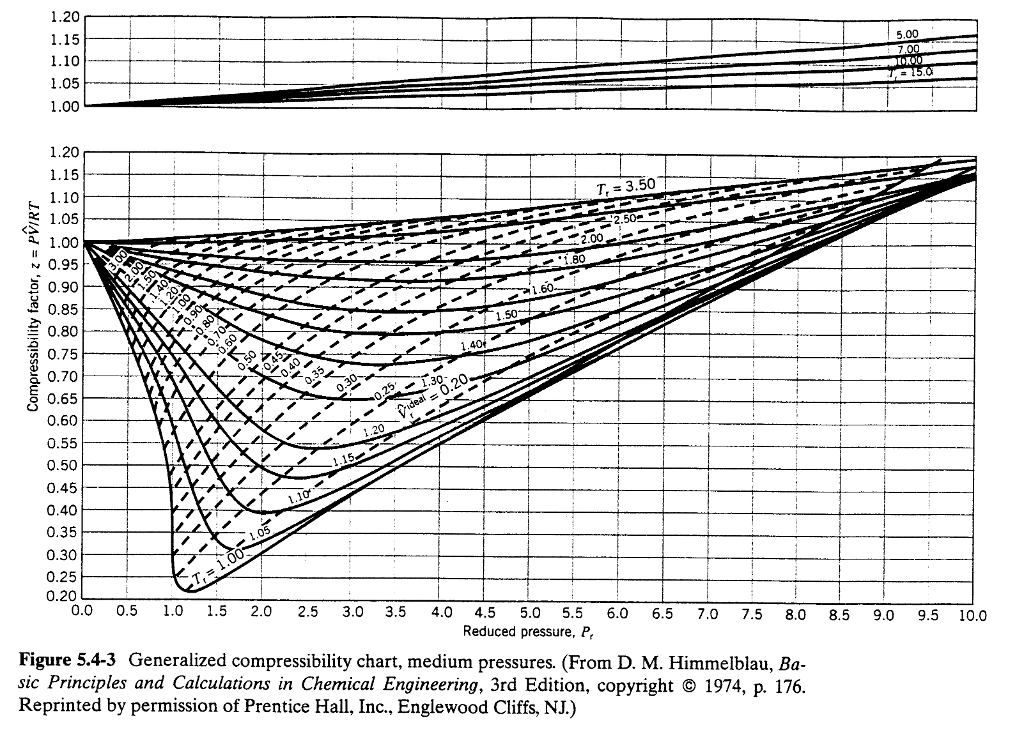
Figures 3.2.1 And 3.2.2 Illustrate The Compressibility Factors Of Hydrogen And Nitrogen, Respectively, Over A.
Thermal Diffusivity Of Nitrogen At 1, 10, 50 And 100 Bara (14.5, 145, 725 And 1450 Psia), And Varying Temperature Given As °C Or °F:
Related Post: