Nitrogen Pressure Temperature Chart
Nitrogen Pressure Temperature Chart - For real gases, the compressibility factor may be very different from one. Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. The relation between these is shown in example 12.7. Web the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask indicates relative amounts. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a. (l) and (s), the normal state of that substance at 1 bar. Popular internal searches in the engineering toolbox. Web hvacr training equipment component sizing psychrometric resource. Web we're going to expend enough energy to put enough molecules into this container to raise the pressure to 100 psig at a temperature of 70°f. Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Web the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask indicates relative amounts. Web calculate a boiling point or pressure using the antoine equation: At room temperature and pressure, liquid nitrogen boils into nitrogen gas. At high temperatures above 1500 k. The relation between these is shown in example 12.7. What is an ideal gas? Web 3 182 people find this calculator helpful. Ideal gas law equation ideal gas constant faqs. For this compound, wtt contains critically evaluated recommendations for: Popular internal searches in the engineering toolbox. Liquid nitrogen is very cold! Web calculate a boiling point or pressure using the antoine equation: The standard states in table a.8 are (g), pure ideal gas at. For this compound, wtt contains critically evaluated recommendations for: The relation between these is shown in example 12.7. Web the thermal conductivity of nitrogen depends on temperature and pressure as shown in the figures and tables below. Web the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask indicates relative amounts.. Web the thermal conductivity of nitrogen depends on temperature and pressure as shown in the figures and tables below. Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of. Web the temperature of liquid nitrogen is −195.79 °c (77 k; The standard states in table a.8 are (g), pure ideal gas at.. The values above apply to undissociated states. The standard states in table a.8 are (g), pure ideal gas at. Ideal gas law equation ideal gas constant faqs. Jahangiri, m., termodynamic properties of nitrogen from the freezing line to 2000 k at pressures to 1000 mpa, j. Web the temperature of liquid nitrogen is −195.79 °c (77 k; If we add more energy, not by compressing more gas but in the form of heat energy, what will happen to the pressure in the container? Icemeister was curious as to how high pressures would be in his nitrogen cylinder when it was stored in a hot ares. Under the same conditions of temperature and pressure, equal volumes of all. (l) and (s), the normal state of that substance at 1 bar. Web compute the values of pressure of a gas for various temperatures using the entered temperature and the known value of pressure at that temperature. Web calculate a boiling point or pressure using the antoine equation: The calculator below can be used to estimate the thermal conductivity of. By abraham solomon friedman nd davidwhite. If we add more energy, not by compressing more gas but in the form of heat energy, what will happen to the pressure in the container? The relation between these is shown in example 12.7. Figures 3.2.1 and 3.2.2 illustrate the compressibility factors of hydrogen and nitrogen, respectively, over a. Web for several substances,. Web the compressibility factor of an ideal gas is exactly one. Online nitrogen thermal conductivity calculator. Web the vapor pressure of liquid nitrogen. Ideal gas law equation ideal gas constant faqs. The standard states in table a.8 are (g), pure ideal gas at. The calculator below can be used to estimate the thermal conductivity of nitrogen at given temperature and atmospheric pressure. Online nitrogen thermal conductivity calculator. Web we're going to expend enough energy to put enough molecules into this container to raise the pressure to 100 psig at a temperature of 70°f. At room temperature and pressure, liquid nitrogen boils into nitrogen gas. For real gases, the compressibility factor may be very different from one. Web hvacr training equipment component sizing psychrometric resource. Streng, 1971 streng, a.g., miscibility and compatibility of some liquid and solidified gases at low temperature, j. Ideal gas law equation ideal gas constant faqs. Web the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the number of particles in each flask indicates relative amounts. Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of. Web the compressibility factor of an ideal gas is exactly one. Web for several substances, such as water, values are shown for two standard states, (1) and (g). Web 3 182 people find this calculator helpful. Web the volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (boyle’s law). Web calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. (l) and (s), the normal state of that substance at 1 bar.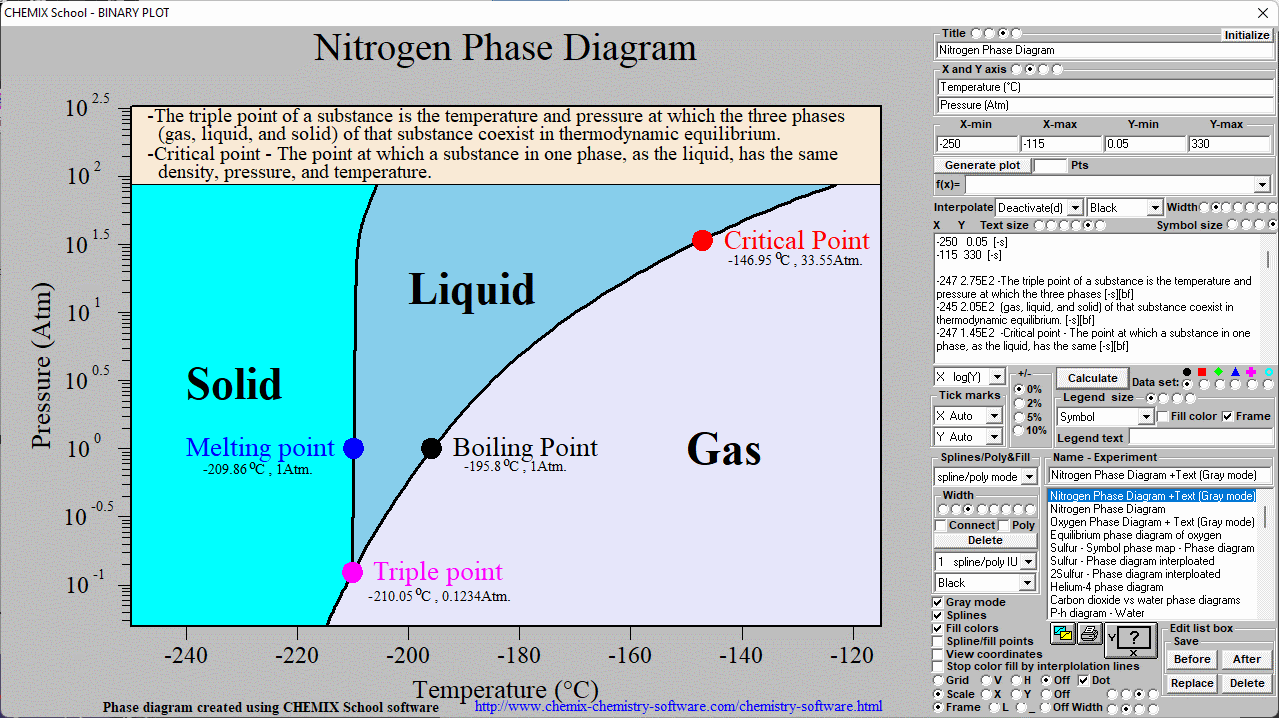
Nitrogen phase diagram

Nitrogen Pressure Temperature Chart

Nitrogen Density and Specific Weight vs. Temperature and Pressure

Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature
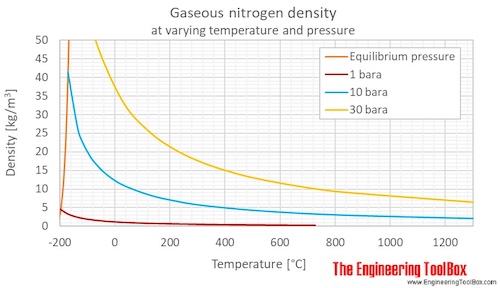
Nitrogen Density and Specific Weight vs. Temperature and Pressure
Nitrogen Pressure Chart A Visual Reference of Charts Chart Master

Nitrogen Thermal Diffusivity vs. Temperature and Pressure

Nitrogen Pressure Temperature Chart A Visual Reference of Charts

Nitrogen Thermal Conductivity vs. Temperature and Pressure

Nitrogen Thermal Diffusivity vs. Temperature and Pressure
Web The Temperature Of Liquid Nitrogen Is −195.79 °C (77 K;
By Abraham Solomon Friedman Nd Davidwhite.
The Calculation Assumes That The Amount And Volume Of Gas Do Not Change.
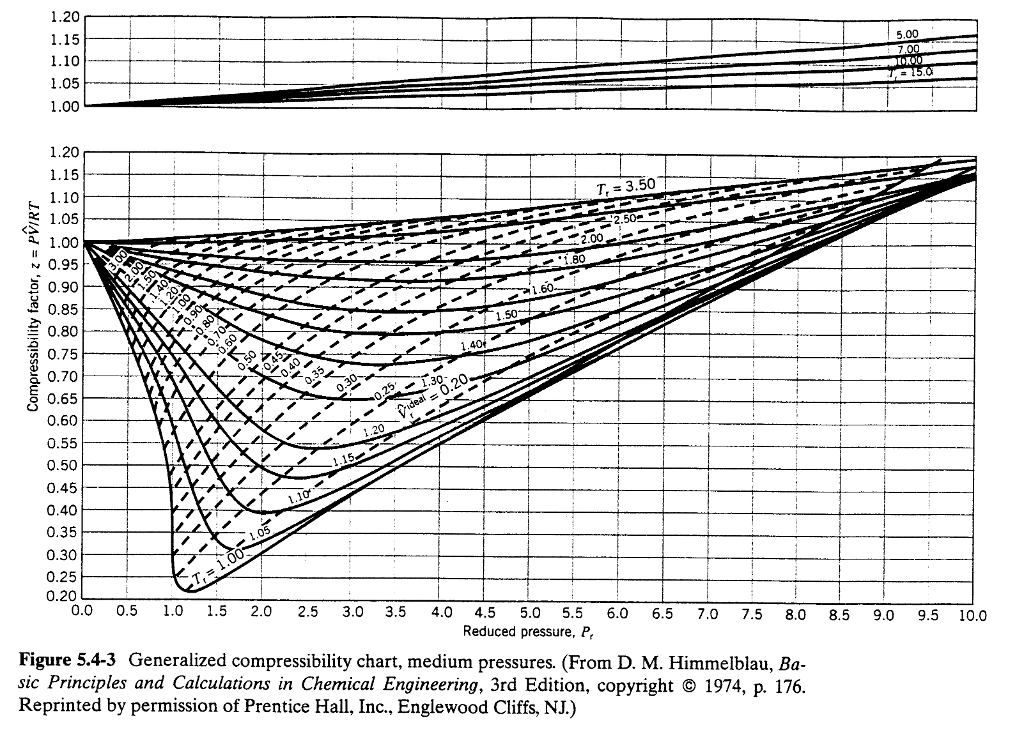
Figures 3.2.1 And 3.2.2 Illustrate The Compressibility Factors Of Hydrogen And Nitrogen, Respectively, Over A.
Related Post: