P Orbital Drawing
P Orbital Drawing - The orbital shows where there is a 95% chance of finding a particular electron. We usually draw p orbitals as figure eights, but we should remember p orbitals are really much fatter than in our usual drawings. Each box represents one orbital, and each arrow indicates one electron. As such, the first shell has no p p orbitals; The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. These orbitals can be categorized on the basis of their size, shape or orientation. This is also due to the history when they were discovered. Web this means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. According to the quantum atomic model, an atom can have many possible numbers of orbitals. P x, p y & p z. Remember that l l must always be less than n n. An s orbital is a sphere. Web subshells are designated by the letters s , p , d , and f , and each letter indicates a different shape. In two dimensions, we draw it as a circle. A p orbital consists of two lobes. Four of them fill the 1s and 2s orbitals. Label the positions of the oxygen nuclei with the symbol o. Web a p orbital is shaped like 2 identical balloons tied together at the nucleus. Because the 2 p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2 p orbitals. It. Web draw an mo cartoon of a sigma bonding orbital formed by the overlap of two p orbitals between two oxygen atoms. Web a p orbital is shaped like 2 identical balloons tied together at the nucleus. This process is the same for the d and f orbitals. This is also due to the history when they were discovered. Remember. Parallel, but not collinear, p orbitals can also interact with each other. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Web this means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up. The unhybridized p orbital) which must contain a pair of electrons used to form the double bond. Because the 2 p subshell has l = 1, with three values of ml (−1, 0, and +1), there are three 2 p orbitals. As the value of l increases, the number of orbitals in a given subshell increases, and the shapes of. As the value of l increases, the number of orbitals in a given subshell increases, and the shapes of the orbitals become more complex. The orbital shows where there is a 95% chance of finding a particular electron. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Web draw an mo cartoon of a sigma bonding orbital formed by the overlap of two p orbitals between two oxygen atoms. Web there are three degenerate 2p orbitals (m l = −1, 0, +1) and the electron can occupy any one of these. Web since the original \(\psi _{p_{+1}}\) and \(\psi _{p_{+1}}\) were both solutions of the schrödinger wave equation, their combinations are also solutions, and so we can visualize atomic orbitals as shapes along the x,y,z axes. In two dimensions, we draw it as a circle. The p sub shell can hold a maximum of six electrons as there are three orbitals. Phosphorus has an atomic number of 15 belongs to group 15 also known as the pnictogens family. Label the positions of the oxygen nuclei with the symbol o. There is a zero probability of finding the electron on that plane. The unhybridized p orbital) which must contain a pair of electrons used to form the double bond. It only has. Earlier, we saw that p orbitals that lie along the same axis can interact to form bonds. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. They would approach each other side by side, above and below the bond axis between the two atoms. This type of hybridization. P orbital has 3 orientations: Web there are three degenerate 2p orbitals (m l = −1, 0, +1) and the electron can occupy any one of these p orbitals. We classified the different orbital into shells and sub shells to distinguish them more easily. Web drawing out the molecular orbitals of each of these systems can be done in a stepwise manner. Parallel, but not collinear, p orbitals can also interact with each other. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Web a p orbital is shaped like 2 identical balloons tied together at the nucleus. There is a zero probability of finding the electron on that plane. Nodal planes, where there is no electron density, are displayed after a short delay. A p orbital consists of two lobes of electron density on either side of the nucleus. Each box represents one orbital, and each arrow indicates one electron. Phosphorus has the symbol p and “it has a concentration in the earth’s crust of about one gram per kilogram”. Orbitals with total angular momentum quantum number l = 1 l = 1 are called p p orbitals. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. An orbital is a space where a specific pair of electrons can be found. College of saint benedict/saint john's university.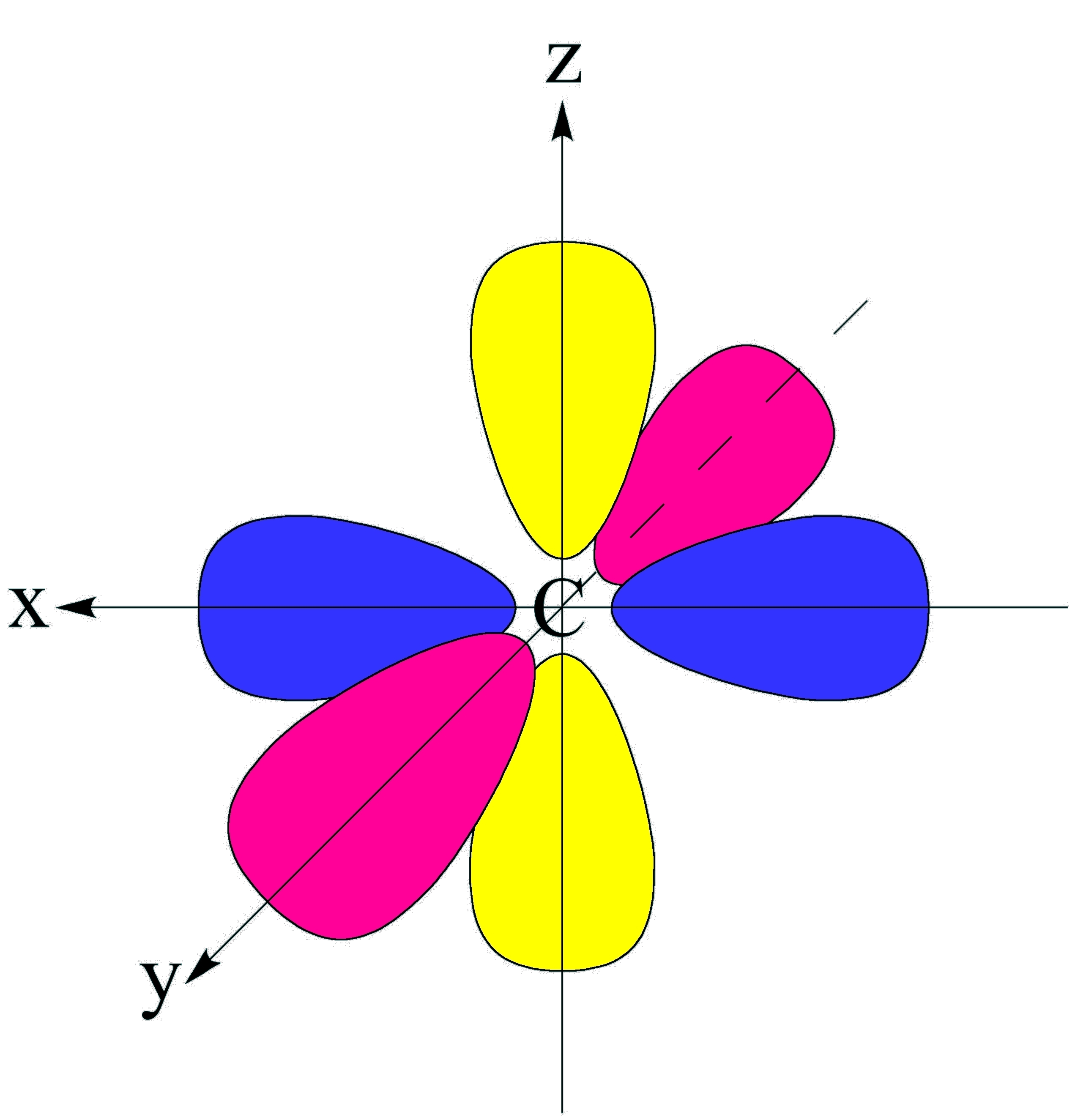
Illustrated Glossary of Organic Chemistry Orbital
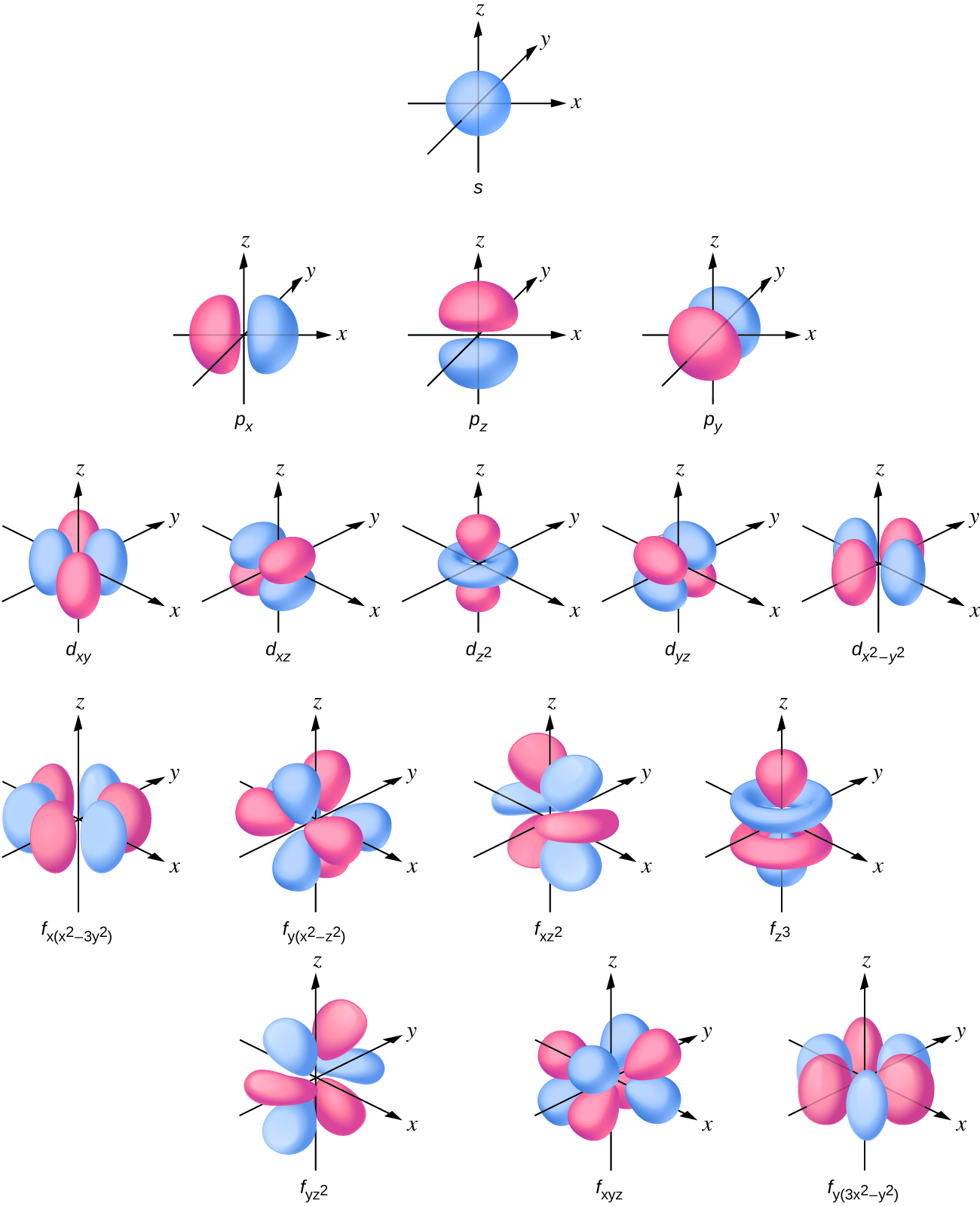
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry

2. What is the shape of p orbital? Brainly.ph
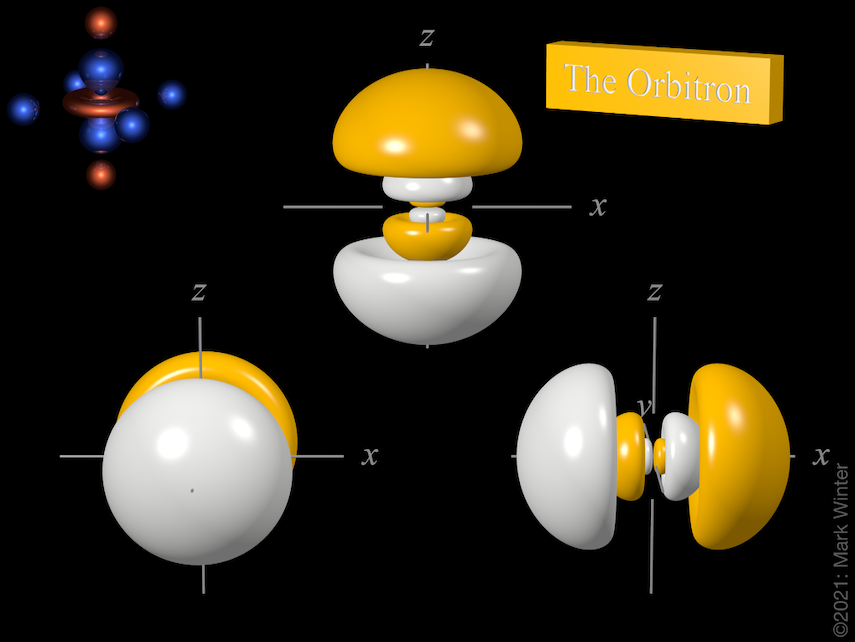
The Orbitron 4p atomic orbitals
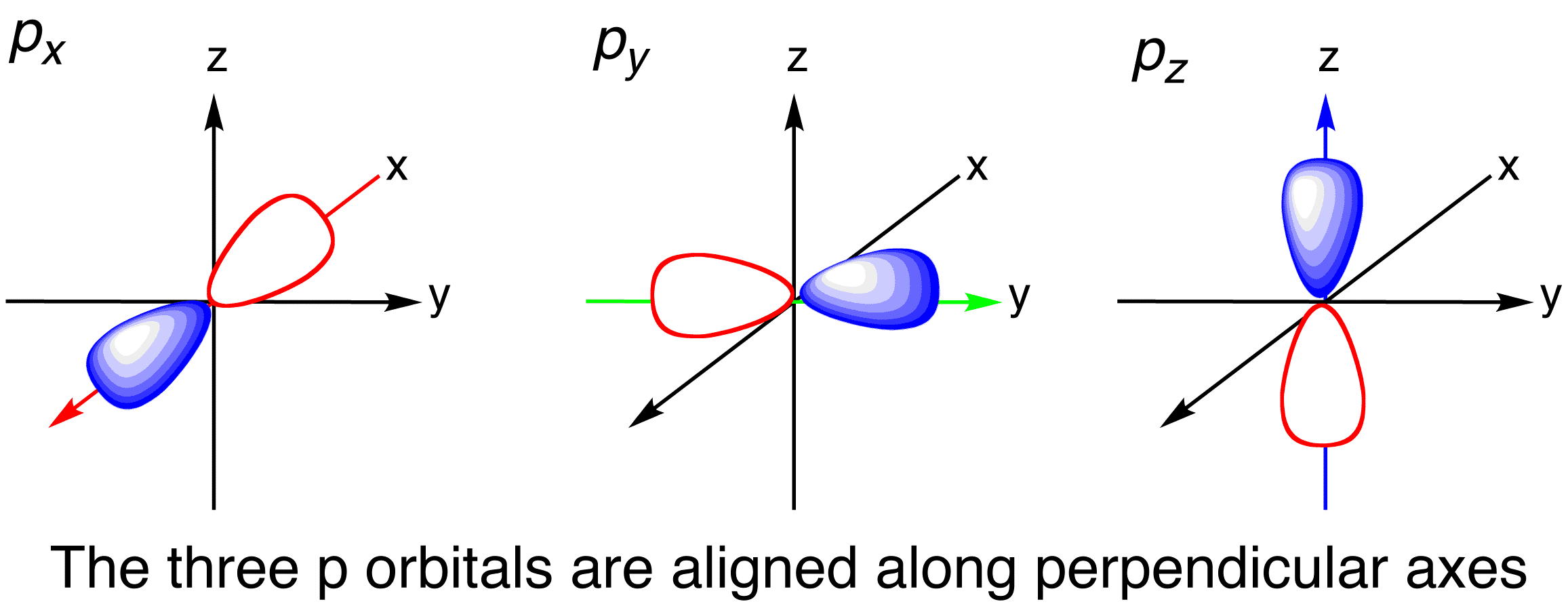
Shape of porbitals in 3D

Shapes of Atomic Orbitals — Overview & Examples Expii

Electron Orbitals

Molecular Orbitals Introductory Chemistry
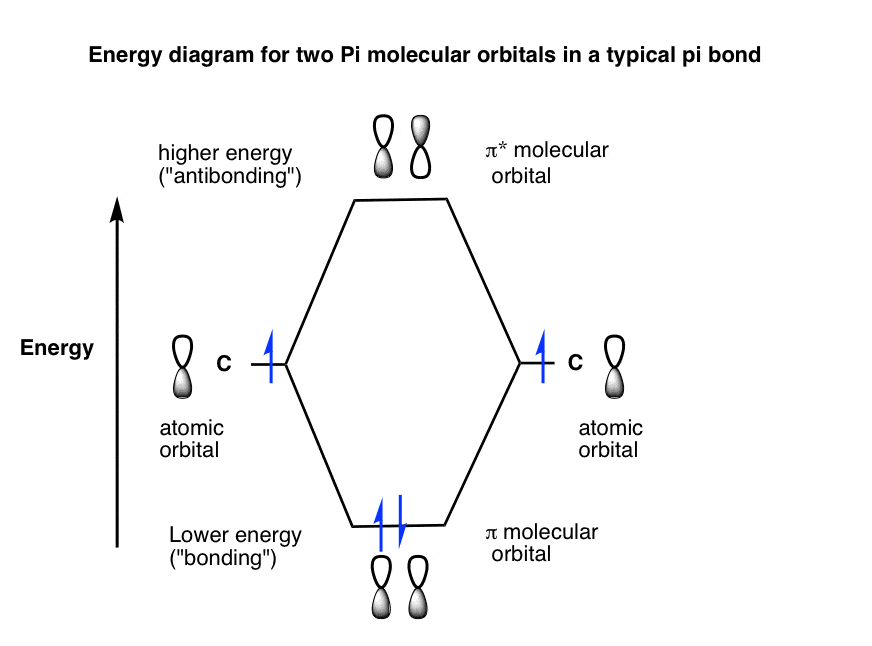
Bonding And Antibonding Pi Orbitals Master Organic Chemistry
[Solved] sketch sigma and pi bond from p orbital Course Hero
In Sp Hybridization, One S Orbital And One P Orbital Hybridize To Form Two Sp Orbitals, Each Consisting Of 50% S Character And 50% P Character.
“Bonding” And “Antibonding” The Full Relationship Energy Diagram.
Carbon (Atomic Number 6) Has Six Electrons.
Want To Join The Conversation?
Related Post: