Periodic Chart With Oxidation Numbers
Periodic Chart With Oxidation Numbers - Oxidation involves an increase in oxidation state. Web charges given to atoms in a molecule in this way are called oxidation numbers. Reduction involves a decrease in oxidation state. Web each element cell contains the atomic number, symbol, name, atomic mass and most common valence charge or oxidation state of each element. Web table of oxidation states of the elements. In our water example, hydrogen is assigned an oxidation number of +1 because each individual hydrogen has lost one electron. Learn how to determine oxidation numbers, along with examples, a diagram, and a chart. Web the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a positive oxidation state) or added to an element (producing a negative oxidation state) to reach its present state. The table is available for download in pdf format for offline printing. Scandium is one of the two elements in the first transition metal period which has only one oxidation state (zinc is the other, with an oxidation state of +2). Web this color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The oxidation number +3 is common to all lanthanides and actinides in their compounds. Rules for assigning oxidation numbers: In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in. Oxidation number is a positive or negative number assigned to an atom according to a set of rules. Web each element cell contains the atomic number, symbol, name, atomic mass and most common valence charge or oxidation state of each element. A lithium atom has one outer shell electron. It has a valence of 1. Elements have an oxidation state. Learn how to determine oxidation numbers, along with examples, a diagram, and a chart. More than one oxidation numbers of an element. Web the oxidation number is the positive or negative number of an atom that indicates the electrical charge the atom has if its compound consists of ions. Oxidation number is a positive or negative number assigned to an. Oxidation number of an atom can be positive or negative or may be zero. In this video, we'll use this method to identify the oxidized and reduced elements in the reaction that occurs between i⁻ and mno₄⁻ in basic solution. First previous 1 next last. Web table of oxidation states of the elements. Web the following chart describes the most. Web this periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights. Zn + 2h+ zn2+ +h2 zn + 2 h + zn 2 + + h 2. Web table of oxidation states of the elements. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. Redox reactions are characterized by a transfer of electrons. In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound. Web interactive periodic table showing names, electrons, and oxidation. Scandium is one of the two elements in the first transition metal period which has only one oxidation state (zinc is the other, with an oxidation state of +2). In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound. Look up chemical element names, symbols,. The oxidation number +3 is common to all lanthanides and actinides in their compounds. Web this periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights. Oxidation number of an atom when an element has combined with the same element. Web periodic table with oxidation numbers. The table is available for. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a. Web what is oxidation number or oxidation state. Redox reactions are characterized by a transfer of electrons. The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. The most common oxidation states. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound. Web this color periodic table contains the number, symbol, name,. Web periodic table with oxidation numbers. Elements have an oxidation state of zero, and atoms in ionic compounds are usually assigned a. Redox reactions are characterized by a transfer of electrons. Web table of oxidation states of the elements. Web determine what is the oxidizing and reducing agents in the following reaction. Web this color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. A lithium atom has one outer shell electron. The oxidation number +3 is common to all lanthanides and actinides in their compounds. To keep track of electrons in a. Oxidation involves an increase in oxidation state. We can use oxidation numbers to keep track of where electrons are in a molecule, and how they move during a reaction. For best results, choose landscape and ‘fit’ for the size option. Web cool periodic table with oxidation numbers and element data and facts. The oxidation number represents how many electrons an atom has gained or lost in a molecule. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. In other words, the oxidation number gives the degree of oxidation (loss of electrons) or reduction (gain of electrons) of the atom in a compound.
Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects
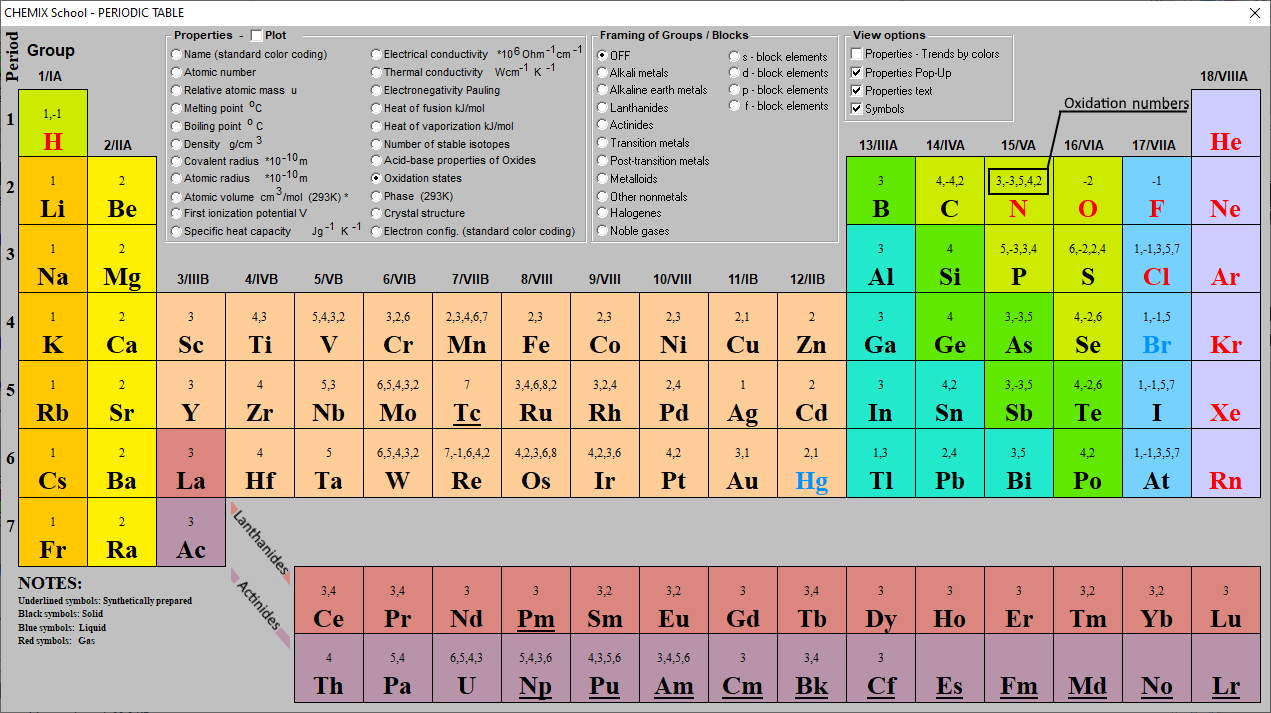
Periodic Table Of Oxidation Numbers Gordon Fastir
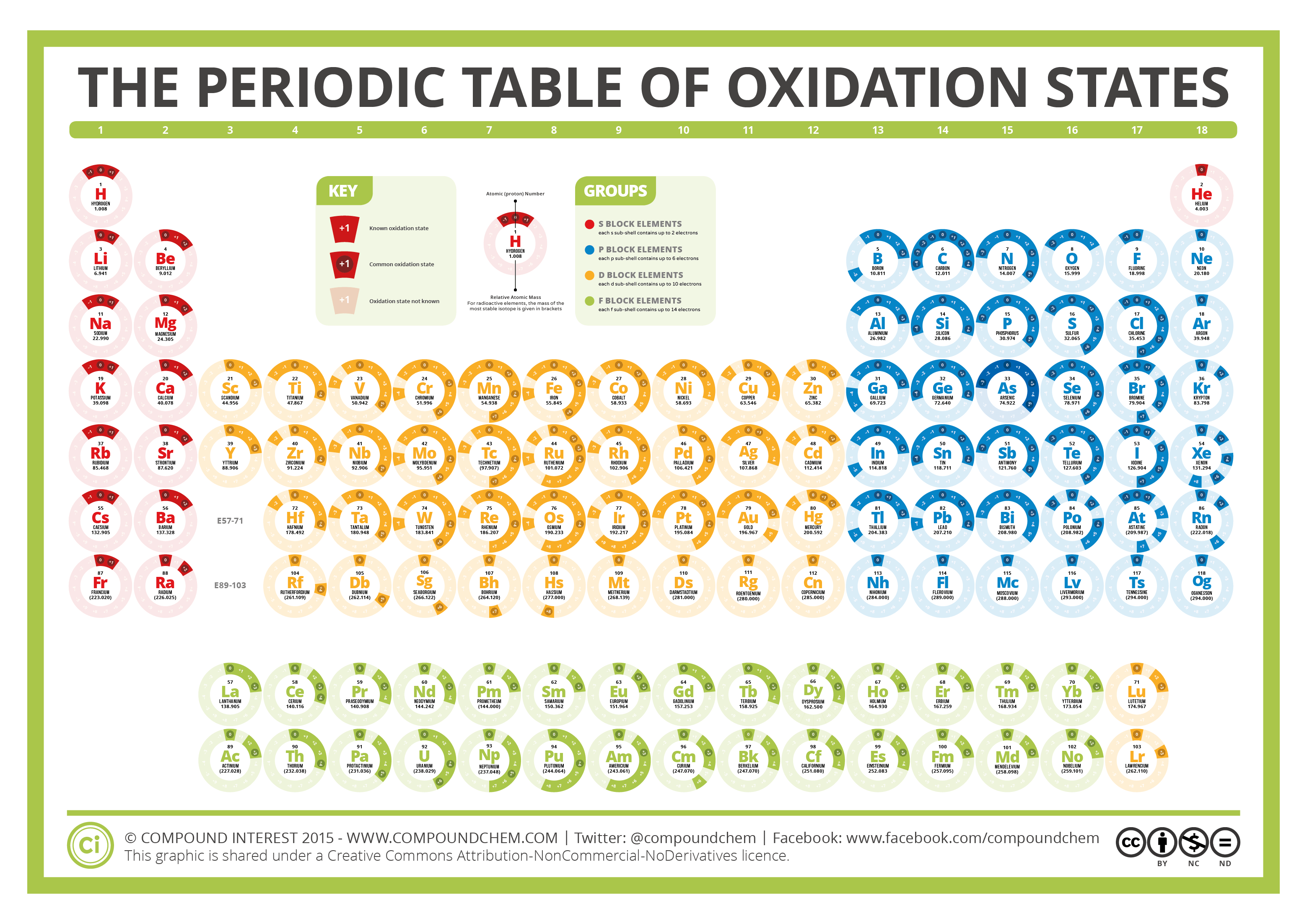
The Periodic Table of Oxidation States Compound Interest
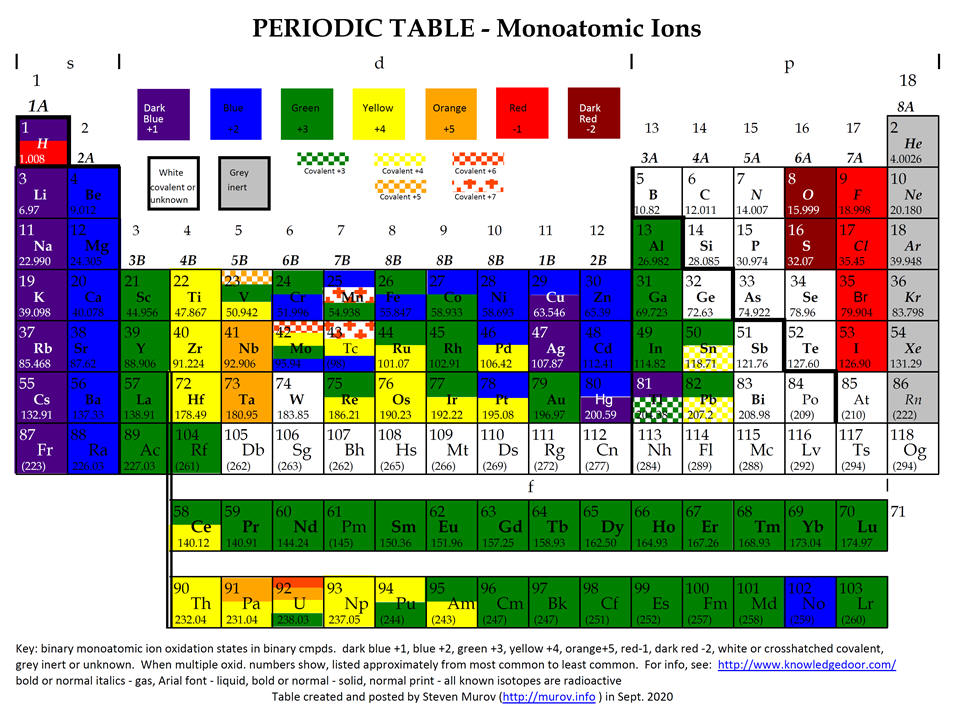
Chemistry Insight
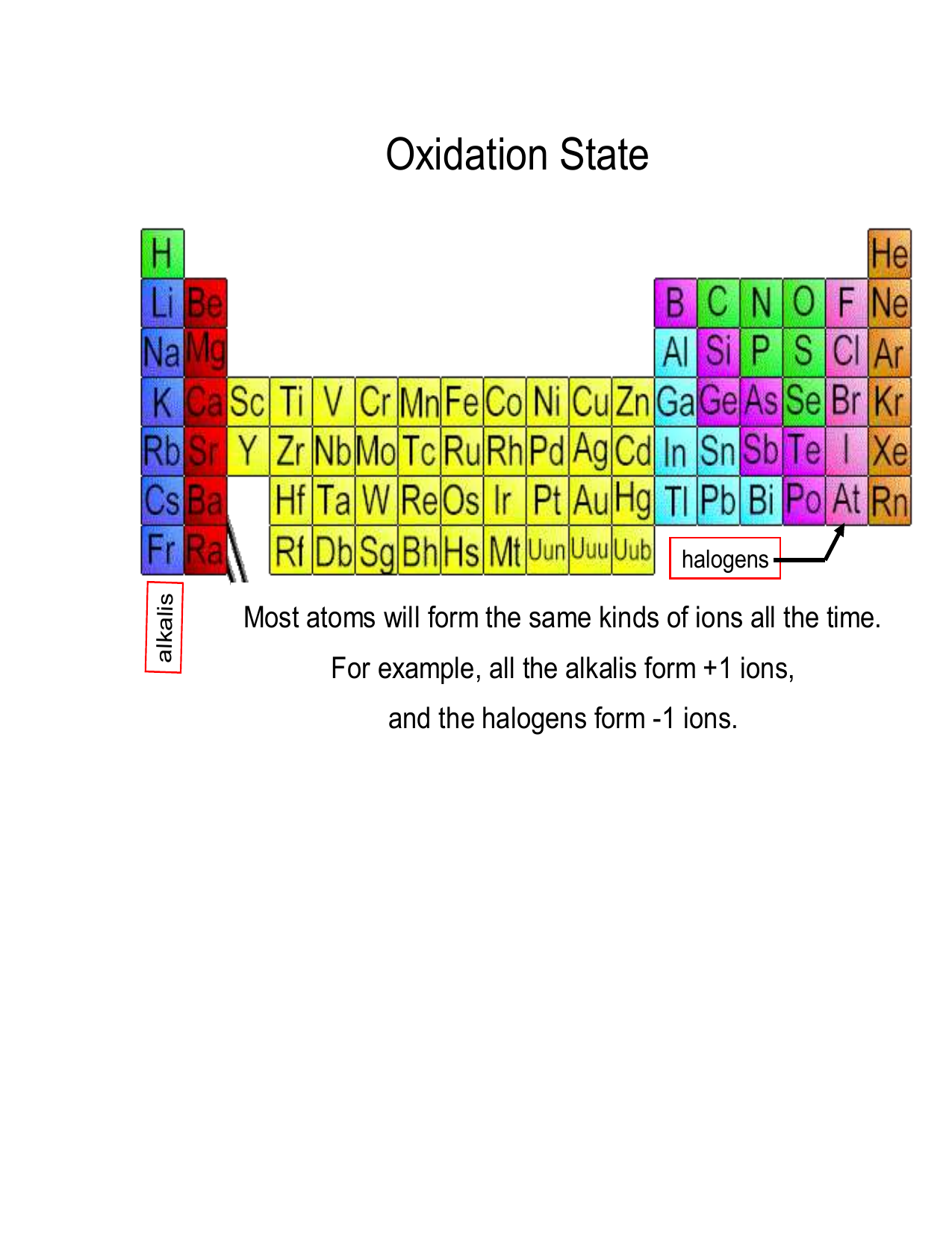
Oxidation Numbers

periodic table oxidation Numbers Diagram Quizlet
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers

Downloadable Periodic Table Oxidation States

Printable Periodic Table With Oxidation Numbers Two Birds Home

Oxidation Number (State) Definition, Rules, How to Find, and Examples
First Previous 1 Next Last.
More Than One Oxidation Numbers Of An Element.
The Oxidation Number Represents The Charge Of An Element In The Compound.
Web The Following Chart Describes The Most Common Oxidation States Of The Period 3 Elements.
Related Post: