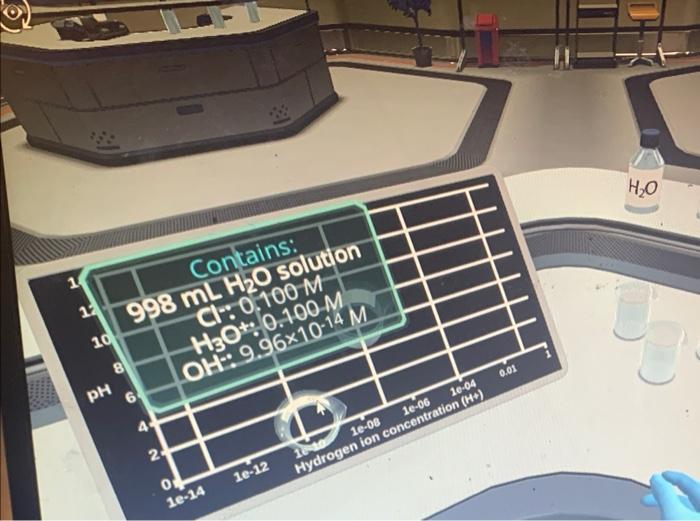
Place The Beaker In The Correct Position On The Chart
Place The Beaker In The Correct Position On The Chart - This problem has been solved! 6 people found it helpful. The beaker should be placed in the first. Anything below 7.0 is acidic, and. Place the beaker in the correct position on the chart. Water potential in the beaker = 0, water potential in the beet core = −0.2 which of. We have 5 beakers with different solutions of nacl. Web le châtelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an. Position the ph sensor in. The mouth of the beaker is rather high, so one must have good coordination to orient the. Place a plastic coated magnet in the beaker. To aid in the saturation of the tlc chamber with solvent vapors, you can line part of the inside of the beaker with. Web the ph scale is often said to range from 0 to 14, and most solutions do fall within this range, although it’s possible to get a ph below. Close the valve, place the funnel back on top, and add. Web the beaker must be clean, dry and at the same temperature as the room and the balance. Create a formula to calculate the ph depending on the h+ concentration ( [h+])! Rinse the spotter with a solvent (e.g. Water potential in the beaker = 0, water potential in. The order of the solutions in order of decreasing. Web position the tip of the pipet below the liquid level in the beaker. Web a beaker can be good for temporarily storing solutions or solvents in when you're working in a lab. 6 people found it helpful. It's easy to add and transfer liquids to, but you can't tell the. Create a formula to calculate the ph depending on the h+ concentration ( [h+])! Web pour solvent into the chamber to a depth of just less than 0.5 cm. The shape of a titration. This problem has been solved! Web now that we have all the information, we can start placing the beakers on the chart. Web in beaker b, what is the water potential of the distilled water in the beaker, and of the beet core? To the sample, add approximately 50 ml of laboratory water and stir continuously with a glass stirring rod for about two minutes. Web le châtelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the. Web position the tip of the pipet below the liquid level in the beaker. The mouth of the beaker is rather high, so one must have good coordination to orient the. Close the valve, place the funnel back on top, and add. Web pour solvent into the chamber to a depth of just less than 0.5 cm. Apply suction and. Reactants \ (\text {a}\) and \ (\text {b}\) will. Web position the tip of the pipet below the liquid level in the beaker. Place a plastic coated magnet in the beaker. Web add 100 ml of distilled water to the beaker. Acetone) if going to use it. The shape of a titration. Web place all five beakers in the correct position on the chart. Web add 100 ml of distilled water to the beaker. Water potential in the beaker = 0, water potential in the beet core = −0.2 which of. Use a utility clamp to suspend a ph sensor on a ring stand. This problem has been solved! Web add 100 ml of distilled water to the beaker. Here’s the best way to solve it. We have 5 beakers with different solutions of nacl. Web the beaker must be clean, dry and at the same temperature as the room and the balance. Here’s the best way to solve it. Acetone) if going to use it. Web spot a dilute sample on the pencil line of the correct lane, making very small spots (\(2 \: Web the beaker must be clean, dry and at the same temperature as the room and the balance. Web now that we have all the information, we can. Web place all five beakers in the correct position on the chart. Web in beaker b, what is the water potential of the distilled water in the beaker, and of the beet core? It's easy to add and transfer liquids to, but you can't tell the volume very. Here’s the best way to solve it. Water potential in the beaker = 0, water potential in the beet core = −0.2 which of. 6 people found it helpful. Web spot a dilute sample on the pencil line of the correct lane, making very small spots (\(2 \: Close the valve, place the funnel back on top, and add. Anything below 7.0 is acidic, and. We have 5 beakers with different solutions of nacl. Web the ph scale is often said to range from 0 to 14, and most solutions do fall within this range, although it’s possible to get a ph below 0 or above 14. Create a formula to calculate the ph depending on the h+ concentration ( [h+])! Acetone) if going to use it. Web position the tip of the pipet below the liquid level in the beaker. This problem has been solved! Position the ph sensor in.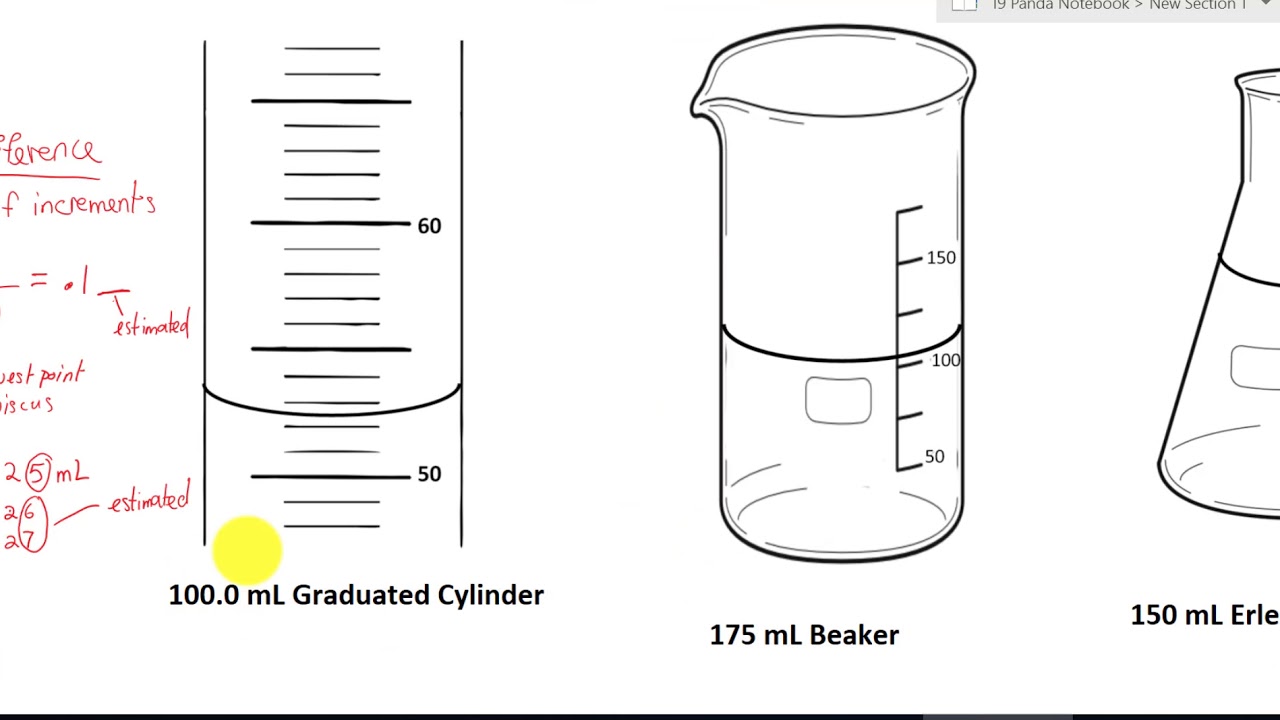
read measurement of graduated cylinder beaker and flask YouTube
Solved Place all five beakers in the correct position on the

Place the Beaker in the Correct Position on the Chart JoyhasRivas
[Solved] Beaker A Beaker B Beaker C Beaker D Beaker E Figure 2 Five
Solved place this beaker on the correct postion on the

Place the Beaker in the Correct Position on the Chart JoyhasRivas
Solved Place all five beakers in the correct position on the
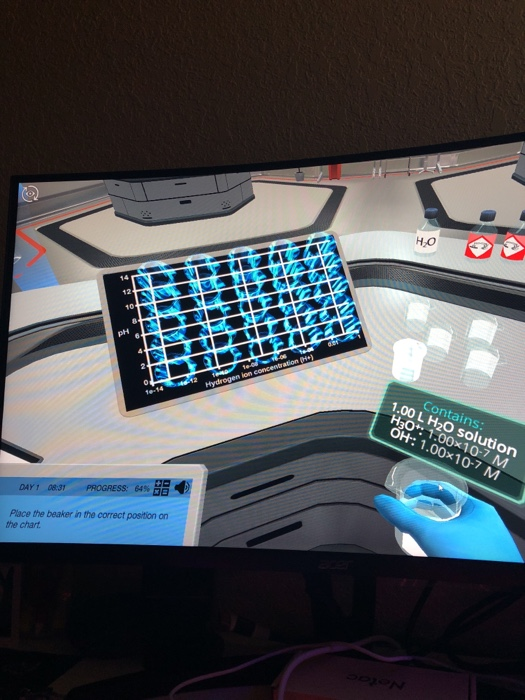
Solved Place the beaker in the correct position on the
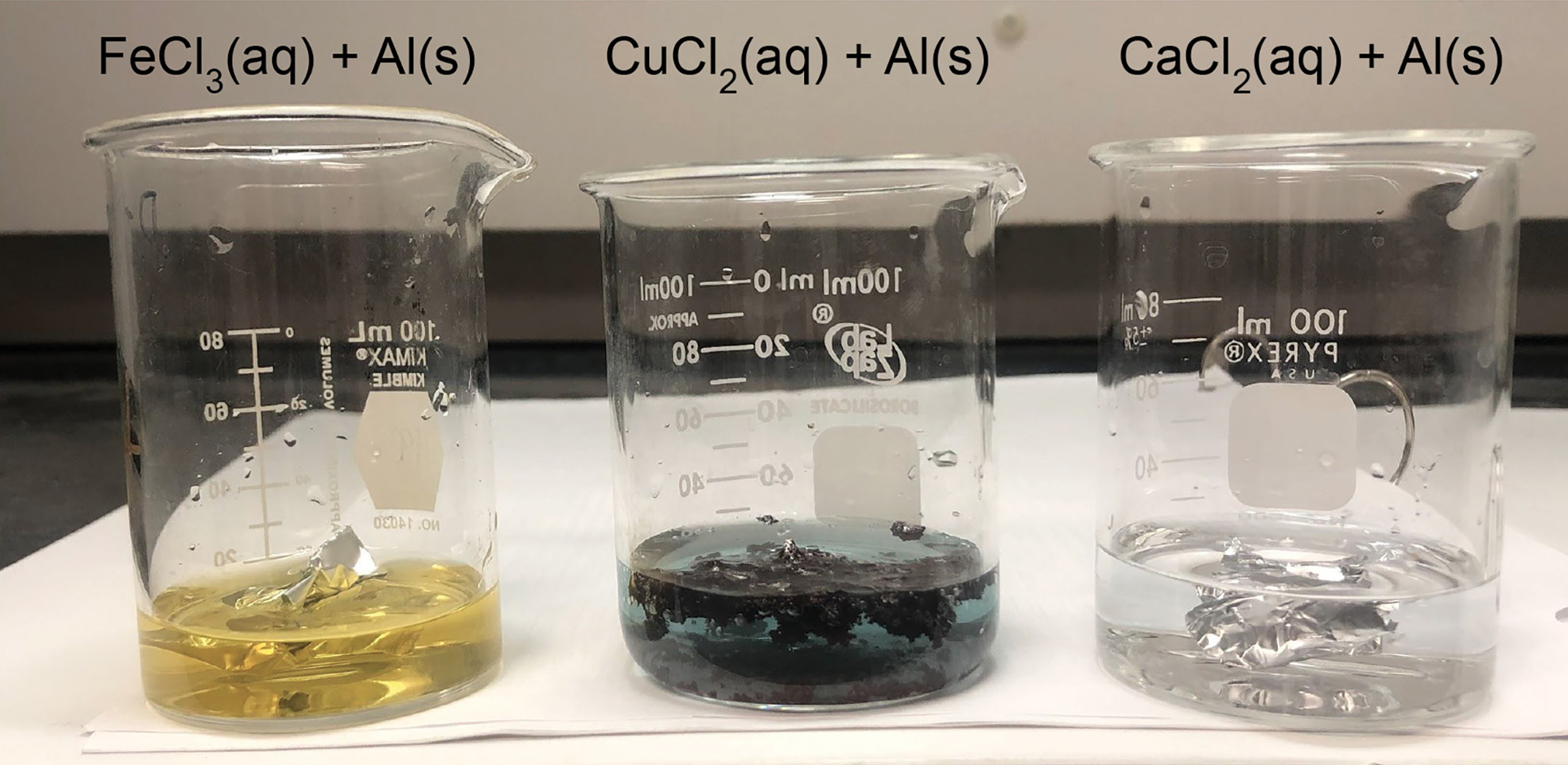
Place the Beaker in the Correct Position on the Chart JoyhasRivas
Solved Place all five beakers in the correct position on the
To The Sample, Add Approximately 50 Ml Of Laboratory Water And Stir Continuously With A Glass Stirring Rod For About Two Minutes.
Web Le Châtelier's Principle States That If A Dynamic Equilibrium Is Disturbed By Changing The Conditions, The Position Of Equilibrium Shifts To Counteract The Change To Reestablish An.
For Example, If We Are Comparing The Volume Of Liquid And The Scale Is Linear,.
The Order Of The Solutions In Order Of Decreasing.
Related Post: