Radius Of Elements Chart
Radius Of Elements Chart - Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Addition of electrons results in an ion that is larger than the parent atom. The relative size of the atoms follows a set of trends on the periodic table. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Ionic radius values range from 31 pm to over 200 pm. Web interactive periodic table showing names, electrons, and oxidation states. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. An atom has no rigid spherical boundary, but it may be thought of as a tiny, dense positive nucleus surrounded by a diffuse negative cloud of electrons. Web the ionic radius is the radius of a monatomic ion of an element within an ionic crystal or half the distance between two bonded gas atoms. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Each atom's size is scaled to the largest element, cesium to show the trend of atom size. The above atomic radii of the elements are the van der waals radius and they are in picometers (pm). Addition of electrons results in an ion that. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Web atomic and ionic radii. The atomic radius is a measure of the distance from the nucleus to the valence electron. This is a periodic table with atomic radius values mentioned on it. Web atomic radius of elements. Atomic radius of an atom is defined as the total distance from nucleus of atom to the outermost orbit in which electron is revolving. Web ionic radius is determined by measuring the atom in a crystal lattice. Web the ionic radius is the radius of a monatomic ion of an element within an ionic crystal or half the distance between. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Elements in the periodic table are organized into periods and groups. The atomic radius of an. You should consult reference 1 for full details, but it is not light reading for most people. Web the periodic table of the elements (including atomic radius) element name. Web this periodic table chart shows the relative sizes of each element. The royal society of chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic. Click the tabs at the top to explore each section. 1 å = 1 × 10−10 m = 100 pm. Elements in the periodic table are organized into periods and groups. The list of atomic radius of elements in periodic table is mentioned below. Web the ionic radius is the radius of a monatomic ion of an element within an. Atomic and ionic radii are found by measuring the distances between atoms and ions in chemical compounds. Web interactive periodic table showing names, electrons, and oxidation states. The royal society of chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Use the buttons above to change your view of the periodic table and view murray robertson’s stunning visual elements artwork. 1 å = 1 × 10−10 m = 100 pm. Each atom's size is scaled to. What is atomic radius on a periodic table? Ionic radius values range from 31 pm to over 200 pm. 1 å = 1 × 10−10 m = 100 pm. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Web the covalent atomic radius (r cov). Atomic radius of an atom is defined as the total distance from nucleus of atom to the outermost orbit in which electron is revolving. This is a periodic table with atomic radius values mentioned on it. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. An atom has no rigid spherical boundary, but it may be thought of as a tiny, dense positive nucleus surrounded by a diffuse negative cloud of electrons. Elements in the periodic table are organized into periods and groups. Web atomic radius chart for elements. Pdf without crop marks | pdf with crop marks. Web atomic radius of all the elements are mentioned in the chart below. Web interactive periodic table showing names, electrons, and oxidation states. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Ionic radius values range from 31 pm to over 200 pm. Atoms consist of a nucleus with positively charged protons and neutral neutrons surrounded by shells of electrons. Definitions of the atomic radius. The relative size of the atoms follows a set of trends on the periodic table. This is a periodic table with atomic radius values mentioned on it. Web ionic radius is determined by measuring the atom in a crystal lattice. What is atomic radius on a periodic table? Web atomic radius of elements. Below mentioned radii are the van der waals radius in picometer (pm)).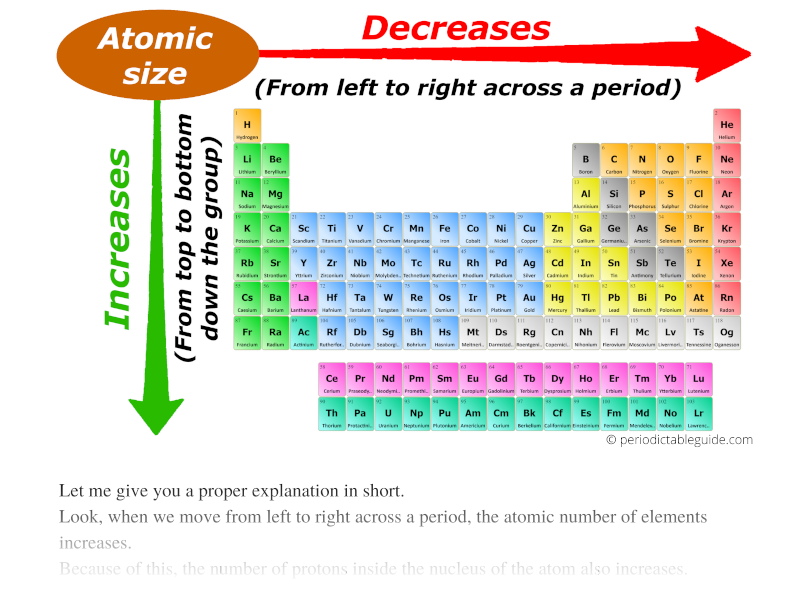
All Periodic Trends in Periodic Table (Explained with Image)
.png)
CK12Foundation
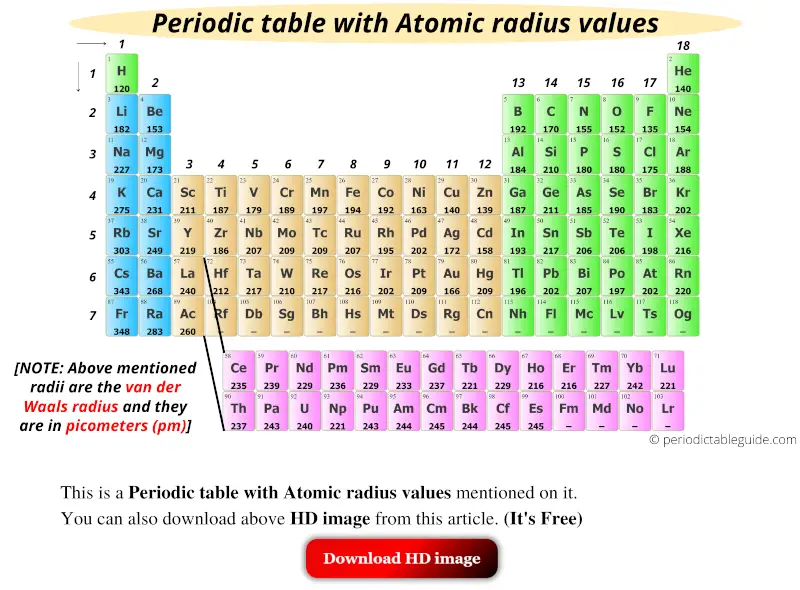
Get the Periodic table with Atomic radius values (Img+Chart)
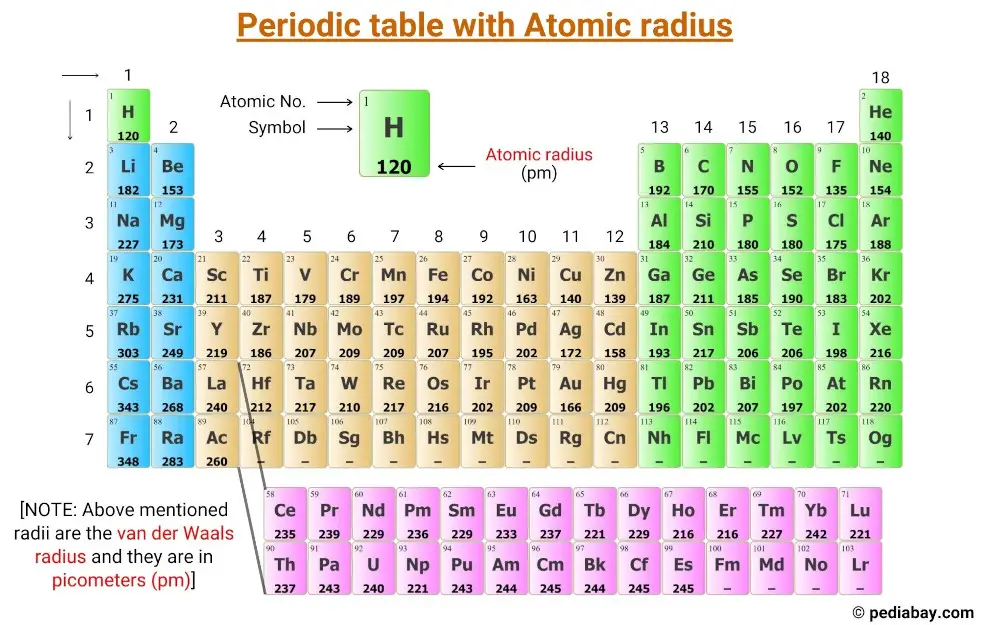
Atomic Radius of Elements (With Periodic table Chart) Pediabay
Periodic Behavior Presentation Chemistry

Measuring Atomic Radius
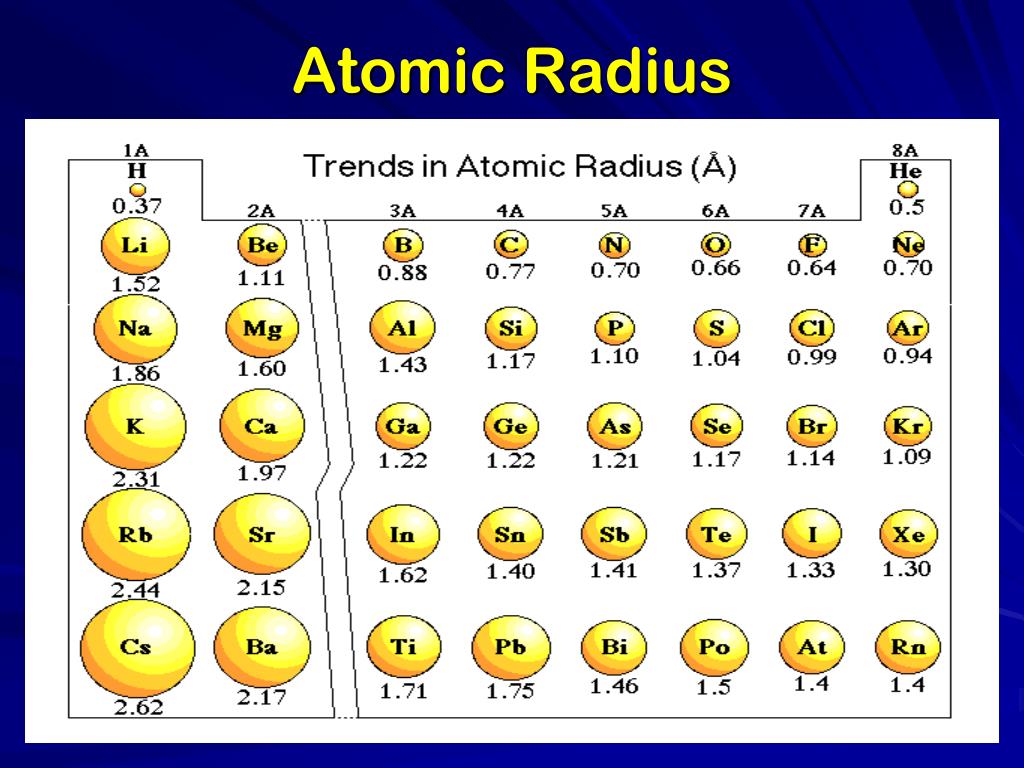
PPT Periodic Table PowerPoint Presentation, free download ID1951146
:max_bytes(150000):strip_icc()/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Size of the Elements on the Periodic Table

Atomic Radius of Elements The Periodic Table

Atomic Radius of Elements
Atomic Radius Is The Measure Of The Distance From The Centre Of The Nucleus To The Outer Electron.
Atomic And Ionic Radii Are Found By Measuring The Distances Between Atoms And Ions In Chemical Compounds.
Web The Periodic Table Of The Elements (Including Atomic Radius) Element Name.
The Atomic Radius Is A Measure Of The Distance From The Nucleus To The Valence Electron.
Related Post:
.PNG)