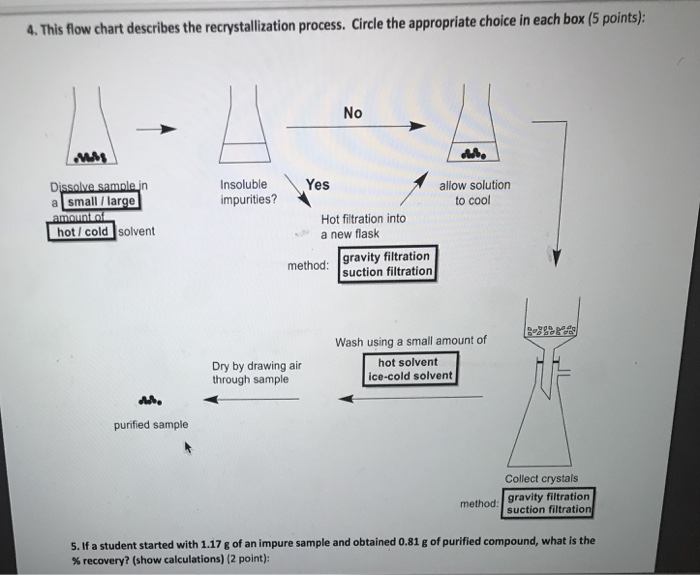
Recrystallization Flow Chart
Recrystallization Flow Chart - Recrystallization (chemistry) in chemistry, recrystallization is a technique used to purify chemicals. Use the symbols s (soluble), ss (somewhat soluble), and i. Institute of fluid flow machinery,. Refer to the flow chart. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. Dissolve the solid in a minimum amount of your boiling solvent to make a nearly saturated solution. “how do you recrystallize a mothball?” techniques checklist. Web the chart shown below should appear in your lab notebook for each of the solutes whose solubility you tested. The recrystallization process is carried. Web recrystallization is based on the different solubility of a solid substance in a solvent at room temperature or when the solvent is hot. In this experiment you will learn to remove impurities from a compound by. Cool the solution to crystallize the product. Refer to the flow chart. Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. Purification of solids by recrystallization. In this experiment you will learn to remove impurities from a compound by. \text{mg}\) of the solid to be crystallized in a. By dissolving a mixture of a compound and. Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. Use the symbols s (soluble),. Recrystallization takes patience, but it’s worth it! Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. The recrystallization process is carried. Web recrystallization is a laboratory technique for purifying solids. Web this involves the following steps. The recrystallization process is carried. Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. The method of purification is based on the principle that. Refer to the. Web the chart shown below should appear in your lab notebook for each of the solutes whose solubility you tested. In this experiment you will learn to remove impurities from a compound by. Institute of fluid flow machinery,. \text{mg}\) of the solid to be crystallized in a. Web recrystallization solvent is one in which the solid has a very high. Institute of fluid flow machinery,. Web this involves the following steps. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. Recrystallization takes patience, but it’s worth it! Web remain in solution during the cold filtration, while the purified solid remains on the filter paper. Web recrystallization is generally accepted to be defined as the formation of a new grain structure in a plastically deformed material. Refer to the flow chart. Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. Cool the solution to crystallize the product. Institute of. The recrystallization process is carried. Purification of solids by recrystallization. Web recrystallization is based on the different solubility of a solid substance in a solvent at room temperature or when the solvent is hot. For most solids, the solubility is proportional. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. Web remain in solution during the cold filtration, while the purified solid remains on the filter paper. Add a small quantity of appropriate solvent to an impure solid. Institute of fluid flow machinery,. Web recrystallization is based on the different solubility of a solid substance in a solvent at room temperature or when the solvent is hot. Procedural summary for. In this experiment you will learn to remove impurities from a compound by. Web recrystallization is a laboratory technique for purifying solids. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. Web the chart shown below should appear in your lab notebook for each of the solutes whose solubility you tested. This. Recrystallization takes patience, but it’s worth it! The method of purification is based on the principle that. Thus, the general steps of recrystallization are as follows. Add a small quantity of appropriate solvent to an impure solid. It involves dissolving the compound in a solvent at high temperature and then allowing it to slowly. Apply heat to dissolve the solid. Recrystallization (chemistry) in chemistry, recrystallization is a technique used to purify chemicals. Purification of solids by recrystallization. Web remain in solution during the cold filtration, while the purified solid remains on the filter paper. Web recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. Web summary of recrystallization steps. The recrystallization process is carried. For most solids, the solubility is proportional. Procedural summary for testing solvents for crystallization. This recrystallization occurs through the. Use the symbols s (soluble), ss (somewhat soluble), and i.Solved 4. This flow chart describes the recrystallization
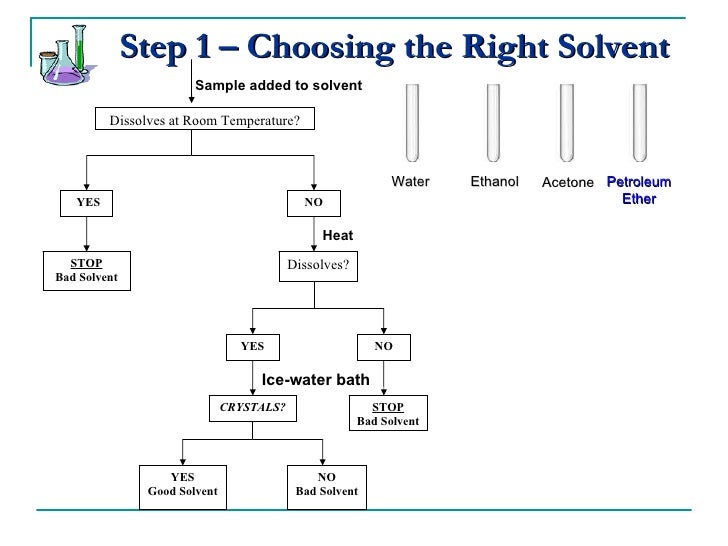
Recrystallization Spring Fall 09
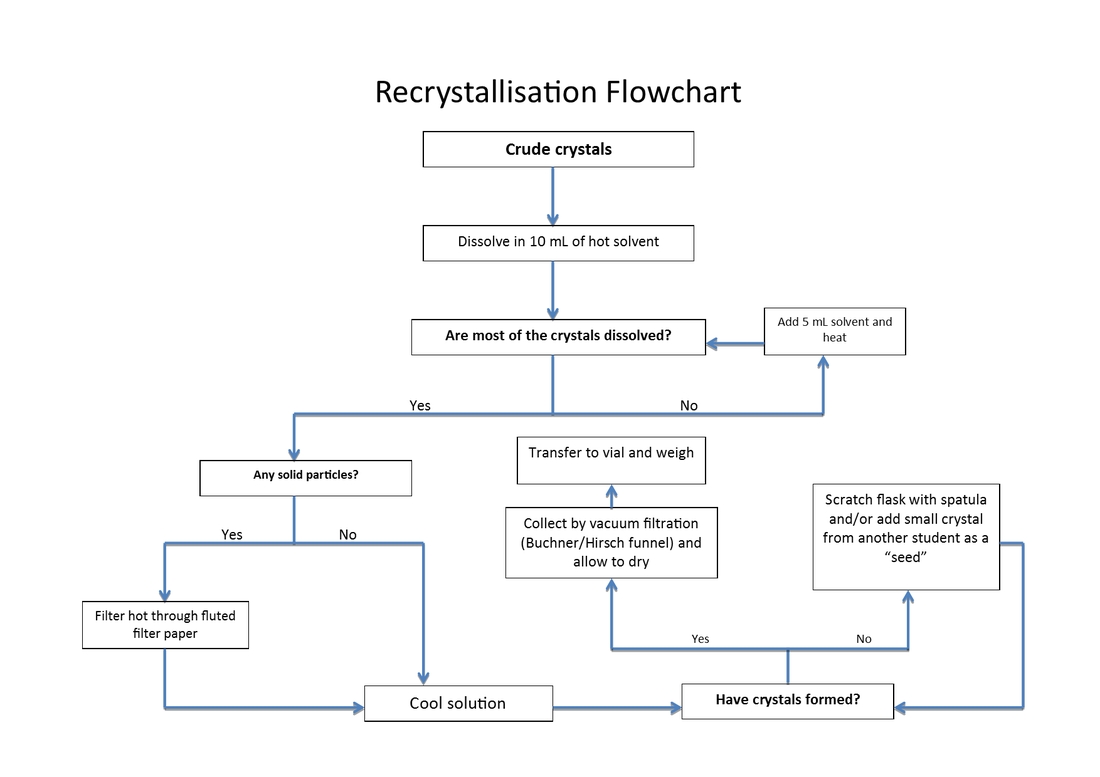
Recrystallisation flowchart
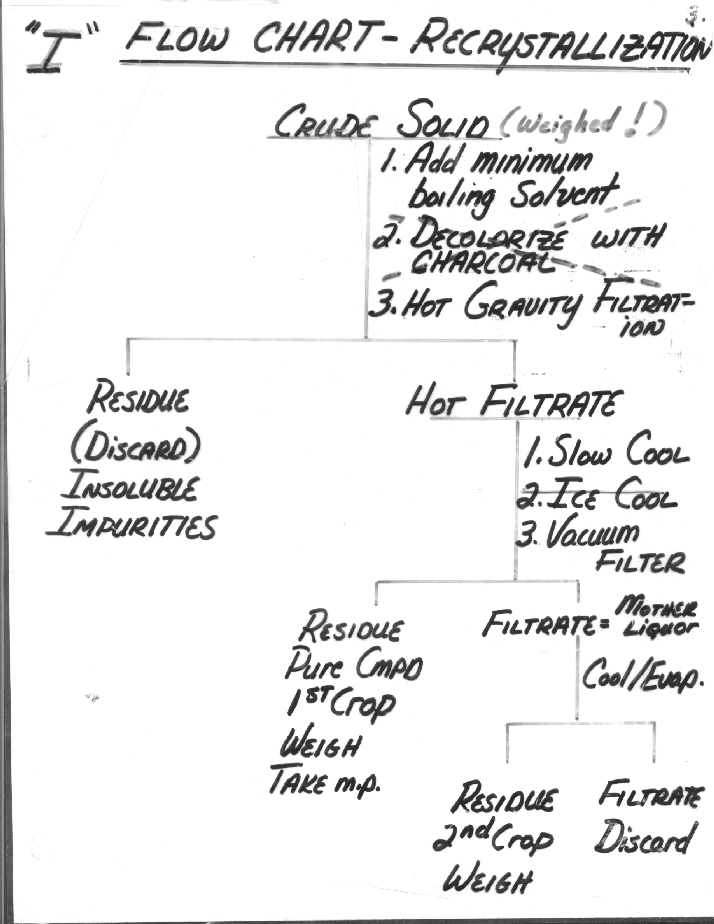
Flowchart Recrystallization
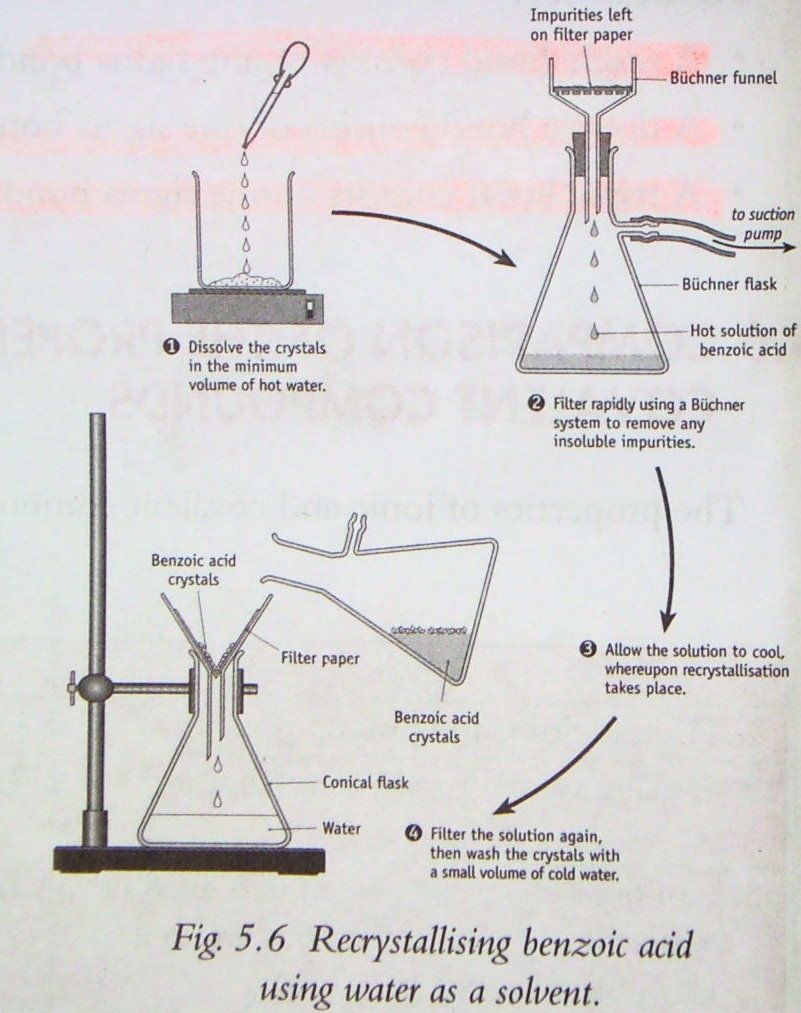
Science St. Gerald's Recrystallisation of Benzoic Acid

Recrystallization
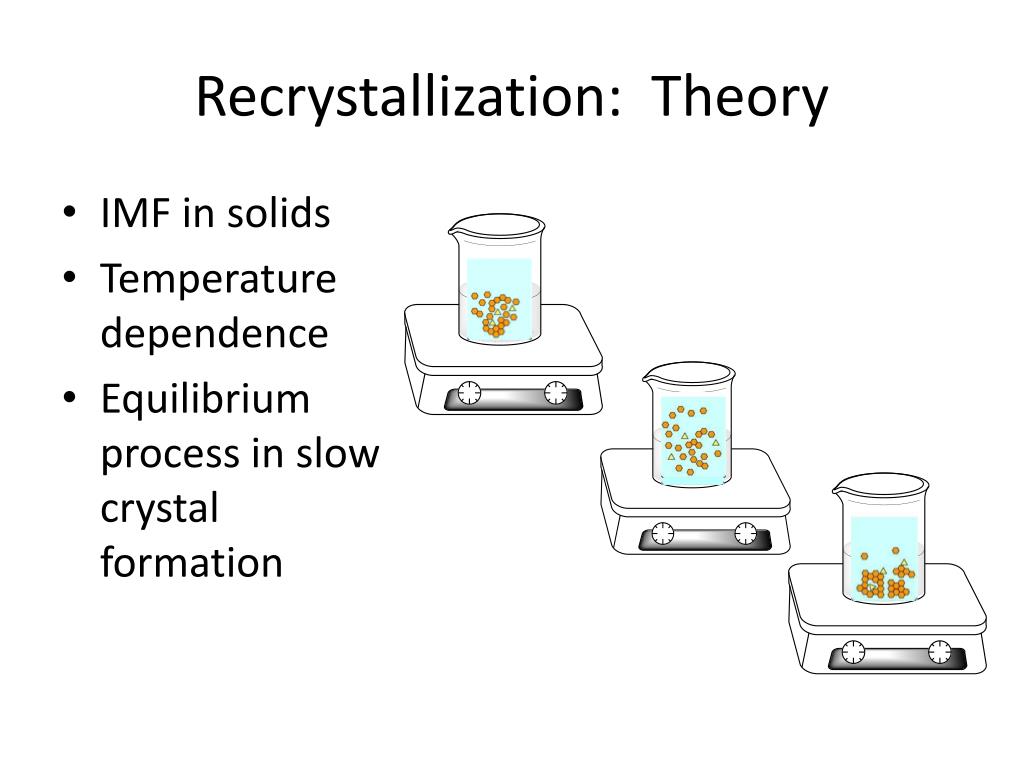
PPT Recrystallization and Melting Point PowerPoint Presentation, free

Flow chart of recrystallization calculation during hot rolling
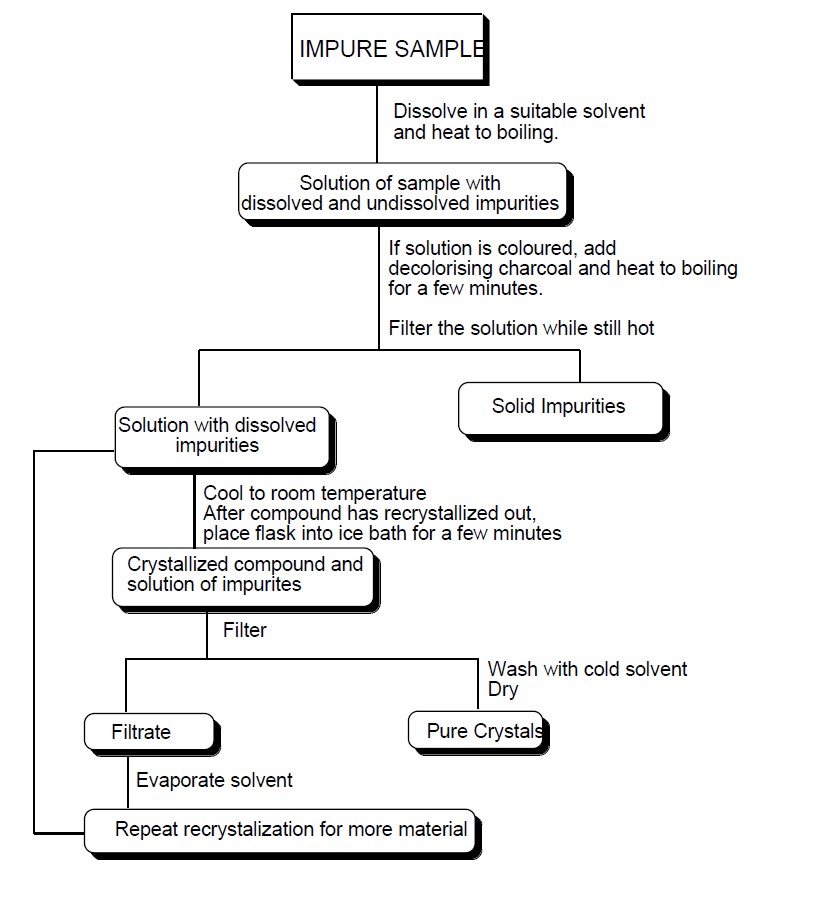
Method of Recrystallization Blog of Dr. D. K. Lokwani
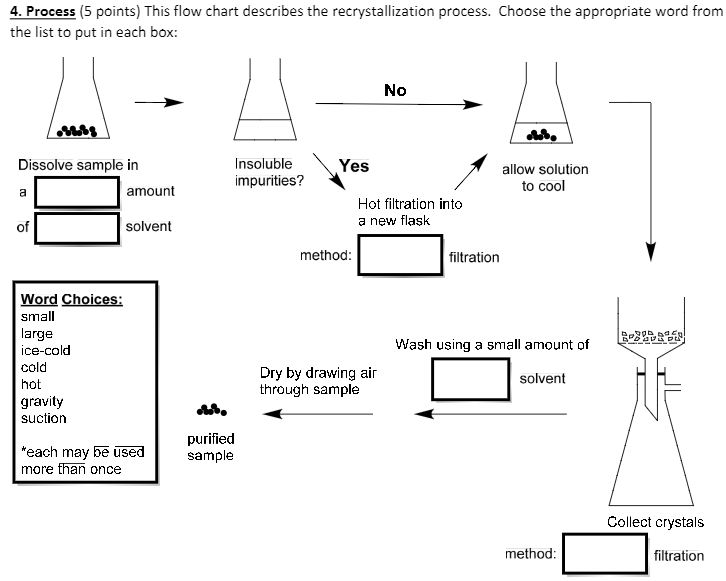
Recrystallization Process
\Text{Mg}\) Of The Solid To Be Crystallized In A.
Dissolve The Solid In A Minimum Amount Of Your Boiling Solvent To Make A Nearly Saturated Solution.
Refer To The Flow Chart.
In This Experiment You Will Learn To Remove Impurities From A Compound By.
Related Post: