Salt Water Freezing Point Chart
Salt Water Freezing Point Chart - But, solubility in a solvent also matters. Basically, the salt concentration in water (salinity) is. How does salt melt ice or snow? Web the document provides a table with the freezing point temperatures and corresponding salt concentrations in brine at 60°f. When seawater freezes, however, the ice contains very little salt because only the water part freezes. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with. Web the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points, respectively, of the solution and the pure solvent. Knowing how different chemicals affect water’s freezing point can help to understand the world, which is filled with water, works. At the eutectic point, the melting or freezing temperature is as low as it will get: Both are proportional to the molality of the solute. Basically, the salt concentration in water (salinity) is. At the eutectic point, the melting or freezing temperature is as low as it will get: Web freezing points for water with freezing mixtures based on salt and ice: Knowing how different chemicals affect water’s freezing point can help to understand the world, which is filled with water, works. Web for saltwater. Web updated on may 05, 2019. Web the freezing point is the temperature at which crystals start to appear when you cool a liquid mixture. Web in water in which the concentration of salt is high, the freezing point can be as low as −6°c. Leveraging a precise formula, it offers insights into the properties and behaviors of saltwater under. Simply put, the salinity of water and its freezing point have an inverse relationship. Salt melts ice essentially because adding salt lowers the freezing point of the water. Web salt and the freezing point of water. (initial observation) in winter time we spread salt in the roads and side walks to melt snow and ice or to prevent ice. Web. Web there is an eutectic composition around 27 wt % nacl or salt dissolved in the water. Web the boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The freezing point of saltwater is thus lower than the freezing point of pure water. But, solubility in a solvent. Knowing how different chemicals affect water’s freezing point can help to understand the world, which is filled with water, works. Leveraging a precise formula, it offers insights into the properties and behaviors of saltwater under certain conditions. Popular internal searches in the engineering toolbox. Is salt creating heat to. Web for saltwater that’s as saturated as it can possibly get. Knowing how different chemicals affect water’s freezing point can help to understand the world, which is filled with water, works. A salt water freezing point calculator is a digital tool designed to determine the change in freezing point when salt (sodium chloride) is added to pure water. If your concentrations of salt are different, then you can scale the boiling. Here is a look at the temperature of the freezing point, the factors that affect it, and whether it’s identical to the melting point. Are you planning to go on a beach vacation and wondering if you need to pack a wetsuit for the colder waters? Salt water freezing point calculator. See also spfs calculator online. Proper brine concentration is. Web for saltwater that’s as saturated as it can possibly get (i.e. Web there is an eutectic composition around 27 wt % nacl or salt dissolved in the water. Salt water freezing point calculator. Eventually, all the ice melts, leaving very cold salt water. Popular internal searches in the engineering toolbox. Web the freezing point is the temperature at which crystals start to appear when you cool a liquid mixture. Eventually, all the ice melts, leaving very cold salt water. Web updated on may 05, 2019. The engineering toolbox privacy policy. In the case of a salt solution with concentrations of salt greater than 23.3%, the solubility curve shows the temperature. Web in water in which the concentration of salt is high, the freezing point can be as low as −6°c. This is when the saltwater is 23.3% salt (by weight). Web fresh water freezes at 32 degrees fahrenheit but seawater freezes at about 28.4 degrees fahrenheit, because of the salt in it. Basically, the salt concentration in water (salinity) is.. Web for saltwater that’s as saturated as it can possibly get (i.e. Web the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points, respectively, of the solution and the pure solvent. Basically, the salt concentration in water (salinity) is. Freezing point, density, specific heat and dynamic viscosity of sodium chloride and water coolant. Freezing point of seawater vs. Web salinity is normally quoted in units ‰ (parts per thousand): Eventually, all the ice melts, leaving very cold salt water. In the case of a salt solution with concentrations of salt greater than 23.3%, the solubility curve shows the temperature at which crystals of salt will appear when you cool a solution of a given concentration. In general electrolytes cause a greater freezing point depression than nonelectrolytes. But, solubility in a solvent also matters. (initial observation) in winter time we spread salt in the roads and side walks to melt snow and ice or to prevent ice. Web updated on may 05, 2019. Do other material do the same? Proper brine concentration is important to fully submerge and freeze foods without issues. How does this melt ice? The good news is you don't need a.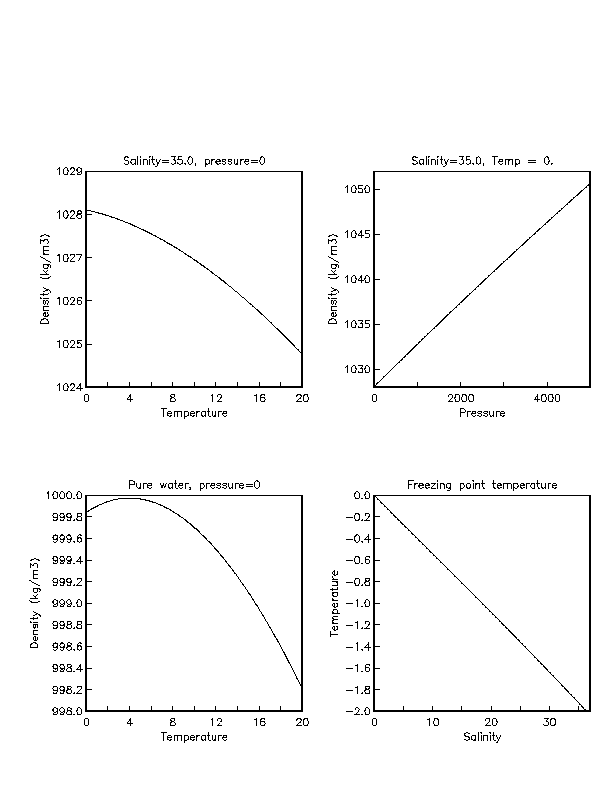
freezing point of salt water karak
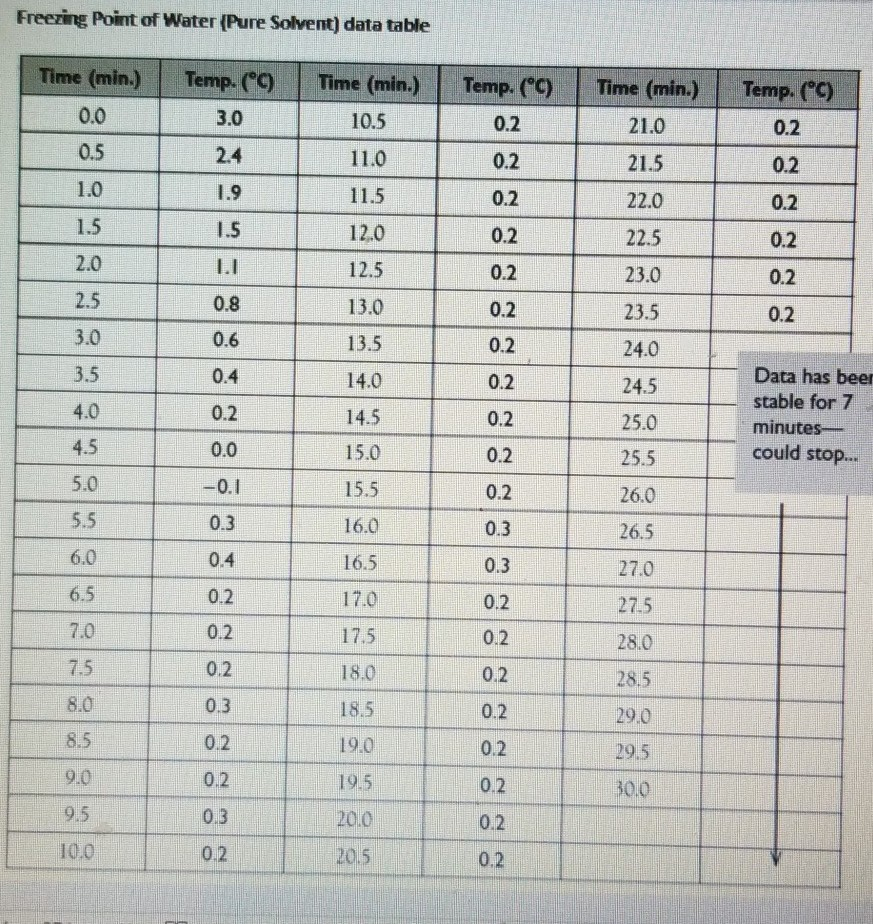
Solved Freezing Point of Water (Pure Solvent) data table

Physics 111 Fundamental Physics I The Physics of Salting Roads
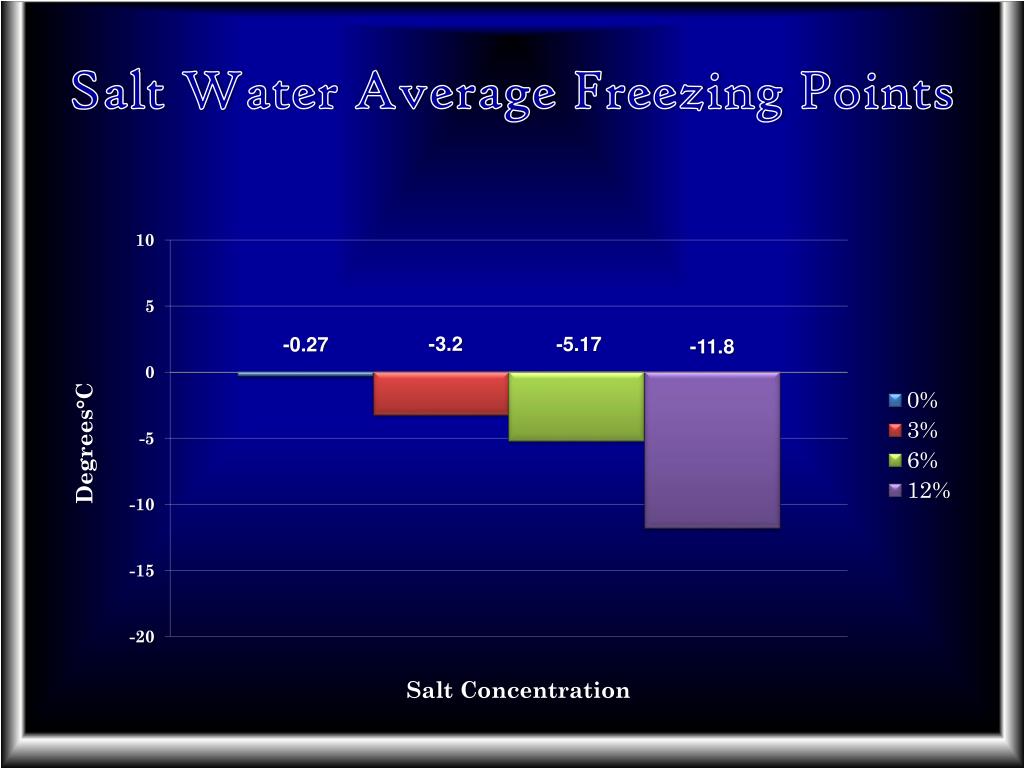
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation
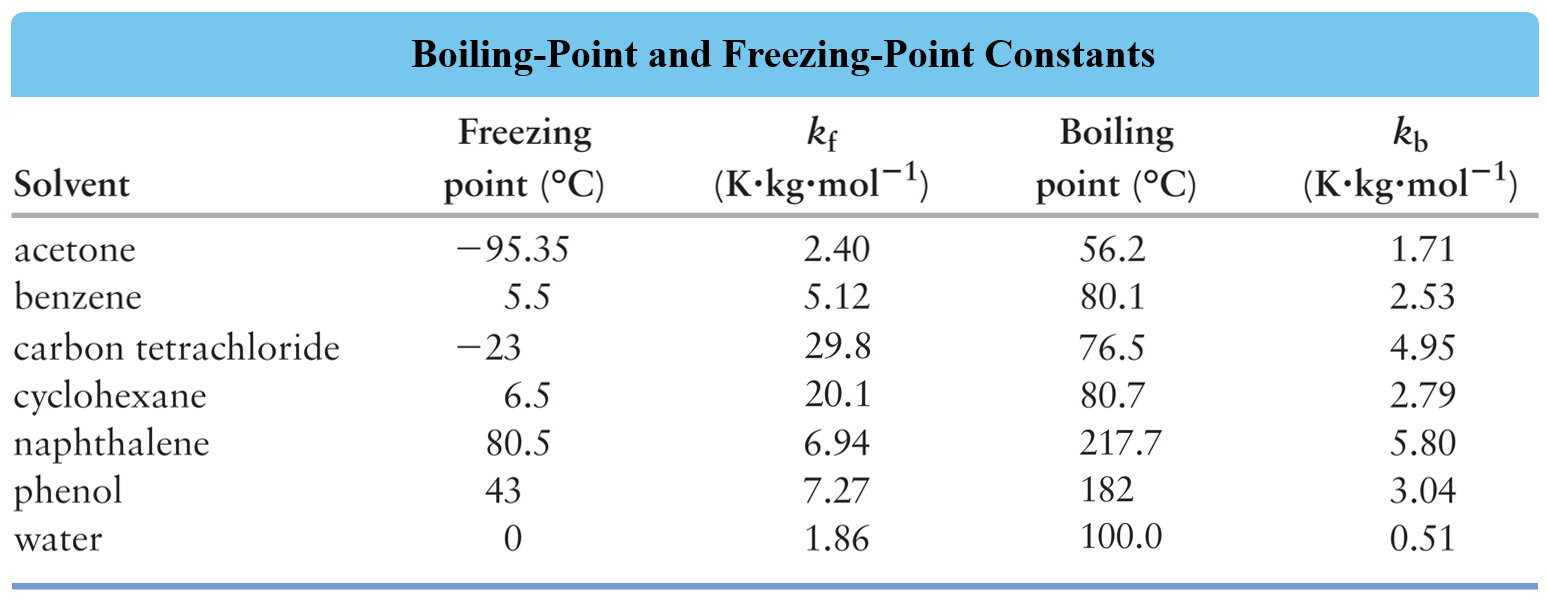
Freezing Point Depression Chemistry Steps

Understand how salt affects the freezing and melting points of water
37+ salt water freezing point calculator TameelSeraphina
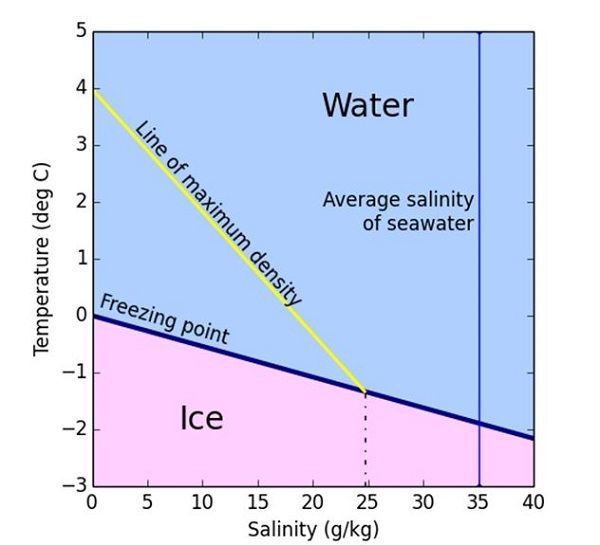
Sea ice an overview Met Office
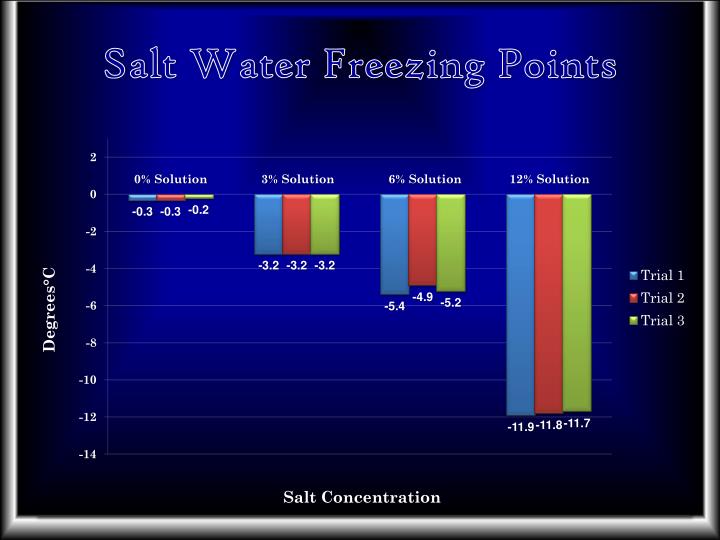
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation
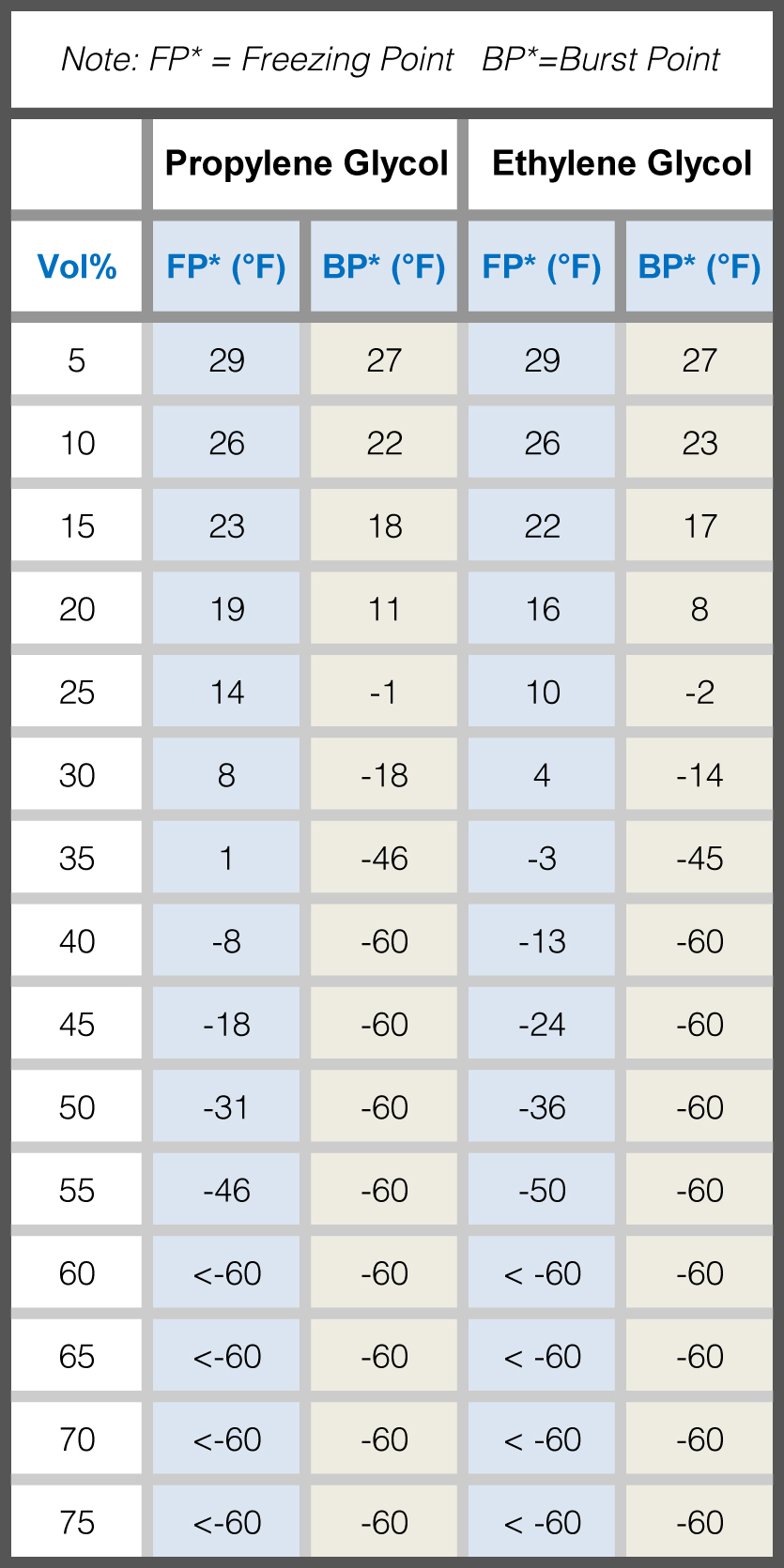
Antifreeze Freezing Point Chart Online Shopping
Web The Freezing Point Is The Temperature At Which Crystals Start To Appear When You Cool A Liquid Mixture.
Leveraging A Precise Formula, It Offers Insights Into The Properties And Behaviors Of Saltwater Under Certain Conditions.
Web The Boiling Point Is Raised By 0.5 Degrees Celsius For Water With 29.2 Grams Of Salt Dissolved In Each Kg Of Water.
Well, It Doesn't, Unless There Is A Little Water Available With The Ice.
Related Post: