Stoichiometry Conversion Chart
Stoichiometry Conversion Chart - Web flowchart of steps in stoichiometric calculations. Web using stoichiometry in conversions. 1 gal = 3.7854 l. I2o5 + brf3 = if5 + o2 + br2. Our tools helps you to know the exact number of moles or grams of the entities involved in a chemical equation. 1 lb = 16 oz = 453.6 g. Web apply a stoichiometric conversion factor to convert between the molar quantities of two substances that participate in a chemical reaction. Use the mole ratio to find moles of other reactant. Web conversion table for problems below. Koh + hcooh = hcook + h2o. 1 lb = 16 oz = 453.6 g. Web conversion table for problems below. Web using stoichiometry in conversions. Use the mole ratio to find moles of other reactant. 1 gal = 3.7854 l. Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of any other reactant or product in the same chemical equation, as explained in the following examples. Apply multiple conversion factors to convert between a molar quantity and a particle count of two substances that participate in a chemical. 1 lb = 16 oz = 453.6 g. Apply multiple conversion factors to convert between a molar quantity and a particle count of two substances that participate in a chemical reaction. Ch3ch2br + ch3oh = ch3ch2och3 + hbr. Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of. Apply multiple conversion factors to convert between a molar quantity and a particle count of two substances that participate in a chemical reaction. C5h12 + h2so4 = c5h11hso3 + h2o. Moles of a is converted to moles of b by multiplying by the molar ratio. I2o5 + brf3 = if5 + o2 + br2. Web we can convert the 3.10. Web conversion table for problems below. Mgsio3 + h2co3 = mgco3 + h2sio3. This tutorial provides a brief overview of dimensional analysis, including conversion. Our tools helps you to know the exact number of moles or grams of the entities involved in a chemical equation. Web flowchart of steps in stoichiometric calculations. Apply multiple conversion factors to convert between a molar quantity and a particle count of two substances that participate in a chemical reaction. Fe2 (so4)3 + agno3 = fe (no3)3 + ag2so4. N2h4 + h2so4 + feso4 = (nh4)2so4 + fe2 (so4)3. Grams of a is converted to moles by multiplying by the inverse of the molar mass. Web using. 1 kg = 1000 g = 2.20462 lb. This tutorial provides a brief overview of dimensional analysis, including conversion. Web using stoichiometry in conversions. Determine where you are starting and finishing step 2: Stoichiometry conversions conversions procedure step 1: We can further expand our understanding of stoichiometry by using the system of ratios we have created as conversions for questions that we are asked in chemistry. Fe2 (so4)3 + agno3 = fe (no3)3 + ag2so4. Web apply a stoichiometric conversion factor to convert between the molar quantities of two substances that participate in a chemical reaction. I2o5 + brf3. Ch3ch2br + ch3oh = ch3ch2och3 + hbr. Web using stoichiometry in conversions. Use the mole ratio to find moles of other reactant. Web n 2 ( g) + 3 h 2 ( g) 2 nh 3 ( g) this equation shows ammonia molecules are produced from hydrogen molecules in a 2:3 ratio, and stoichiometric factors may be derived using any. Use the mole ratio to find moles of other reactant. September 13th, 2011 | author: Web flowchart of steps in stoichiometric calculations. Web conversion table for problems below. Web we can convert the 3.10 grams of h a 2 so a 4 to moles using the molar mass of h a 2 so a 4 ( 98.08 g / mol. Web n 2 ( g) + 3 h 2 ( g) 2 nh 3 ( g) this equation shows ammonia molecules are produced from hydrogen molecules in a 2:3 ratio, and stoichiometric factors may be derived using any amount (number) unit: 1 lb = 16 oz = 453.6 g. C2h6 + ch3cooh = c4h8o2 + h2o. 1 cm 3 = 1ml. Koh + hcooh = hcook + h2o. Web conversion table for problems below. 3.10 g h 2 so 4 × 1 mol h 2 so 4 98.08 g h 2 so 4 = 3.16 × 10 − 2 mol h 2 so 4. Web flowchart of steps in stoichiometric calculations. Web we can convert the 3.10 grams of h a 2 so a 4 to moles using the molar mass of h a 2 so a 4 ( 98.08 g / mol ): 1 gal = 3.7854 l. Stoichiometry conversions conversions procedure step 1: I2o5 + brf3 = if5 + o2 + br2. 2 nh 3 molecules 3 h 2 molecules or 2 doz nh 3 molecules 3 doz h 2 molecules or 2 mol nh 3 molecules 3 mol h 2 molecules. September 13th, 2011 | author: Web the conversion factors are used to calculate the unknown quantity in the mole from the known quantity in the mole of any other reactant or product in the same chemical equation, as explained in the following examples. Grams of a is converted to moles by multiplying by the inverse of the molar mass.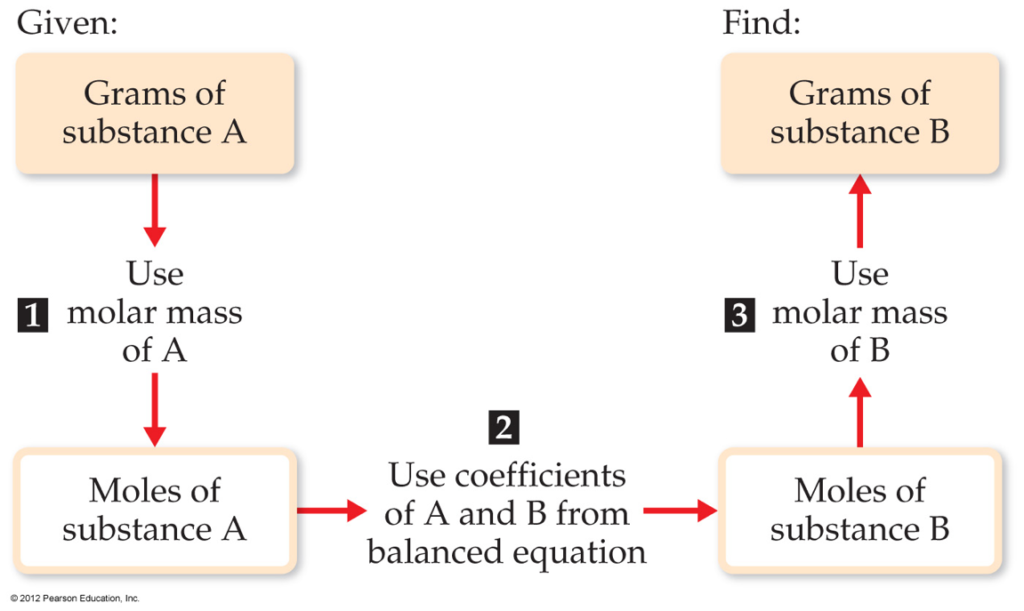
Stoichiometry Chemistry Activities

Stoichiometry Lessons TES chemistry & physics Pinterest
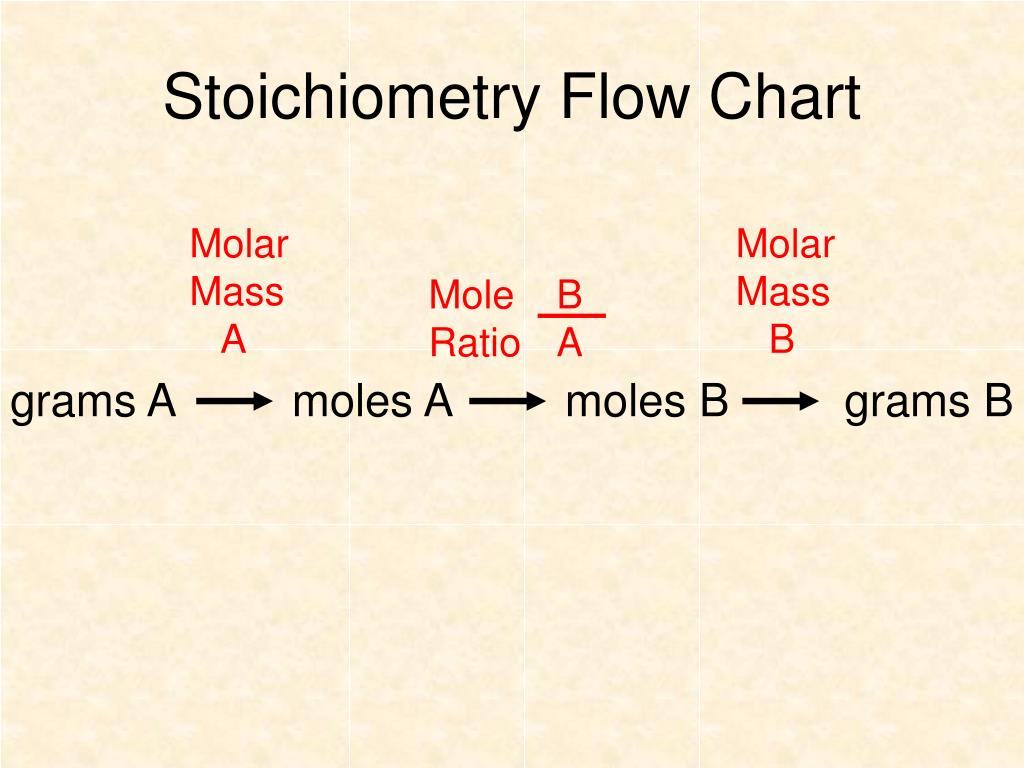
PPT Chapter 12 Stoichiometry PowerPoint Presentation, free download
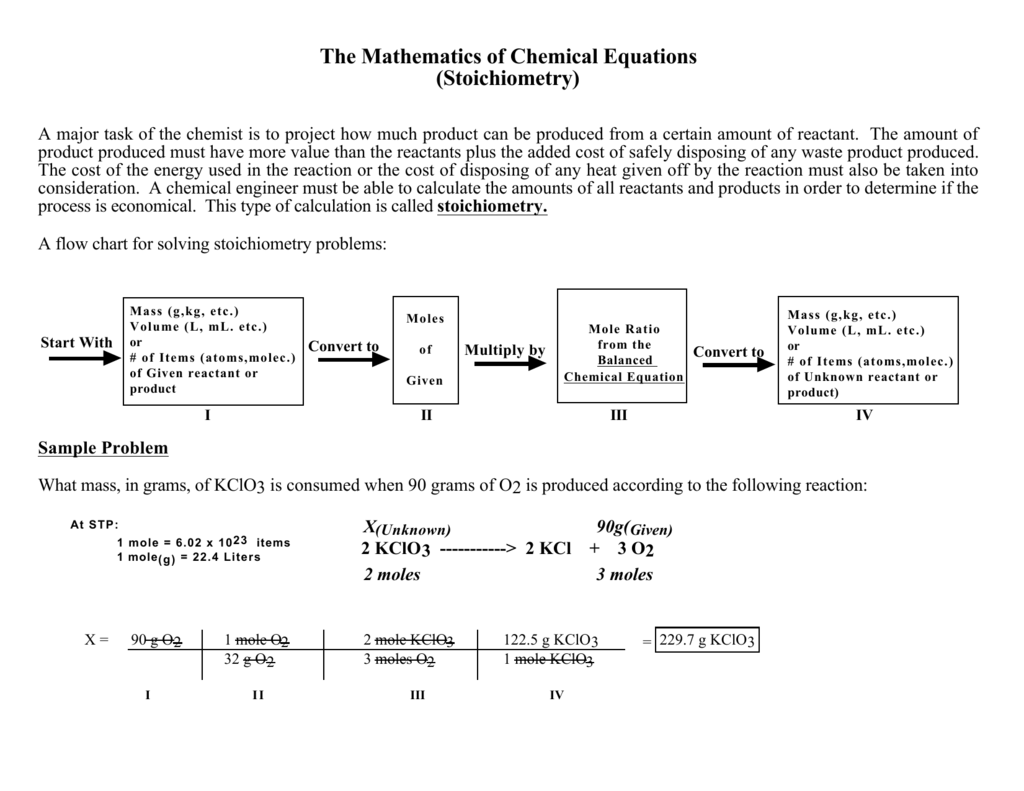
Stoichiometry Chart

Ch. 5 Stoichiometry
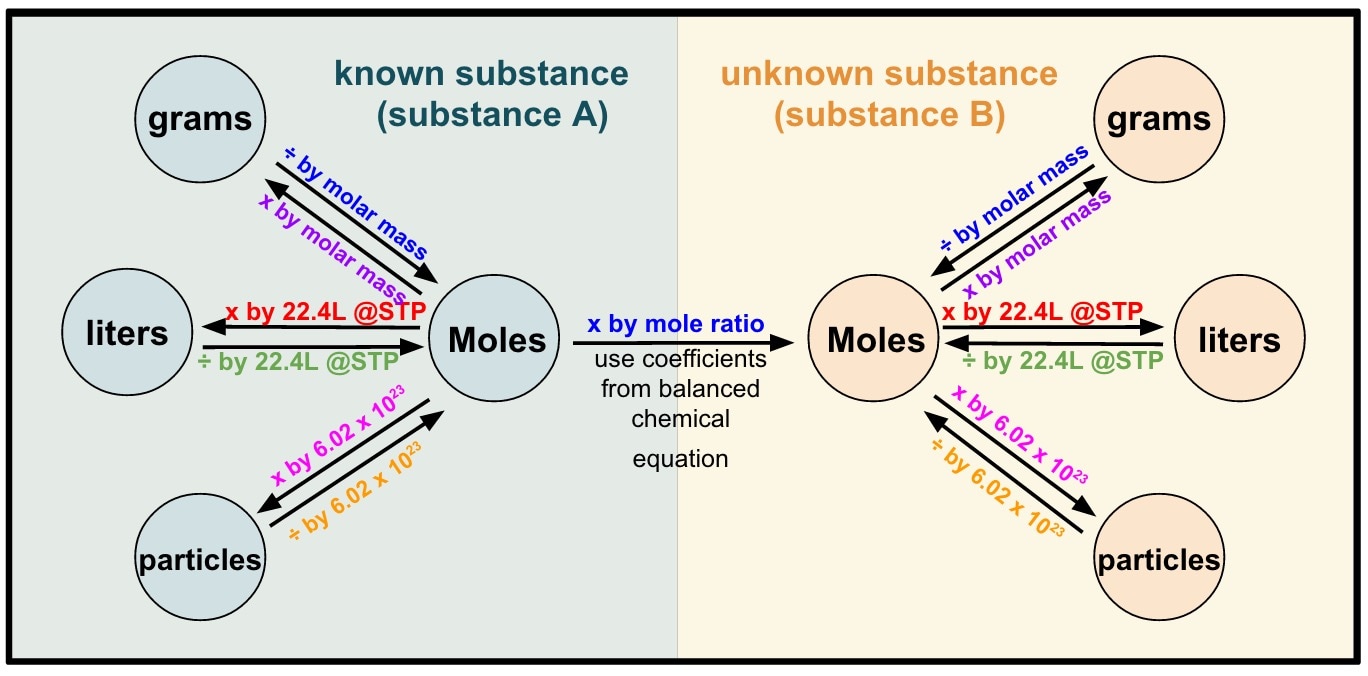
Stoichiometry Review Mr. Siemianowski Eisenhower High School
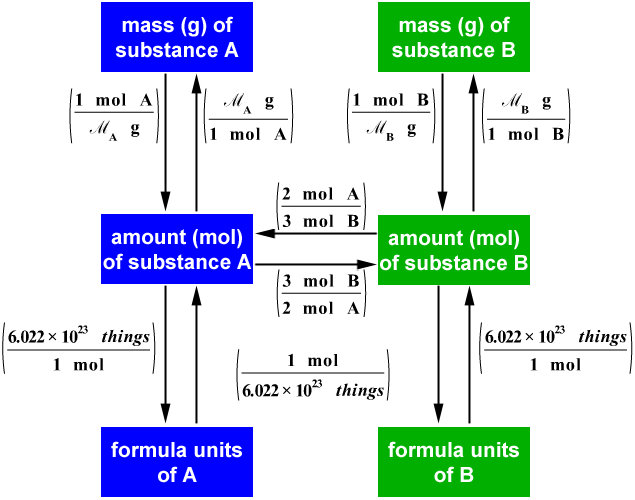
C&J&S&B's Class Chemisty ) What is Stoichiometry?
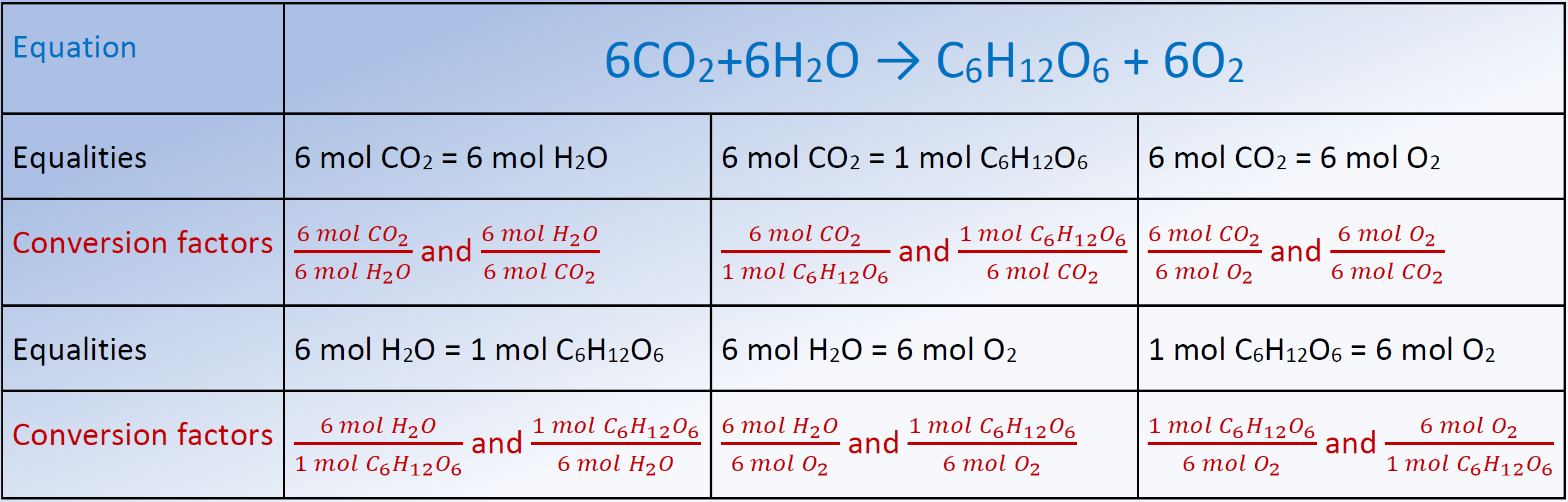
Chemistry Conversion Chart Moles
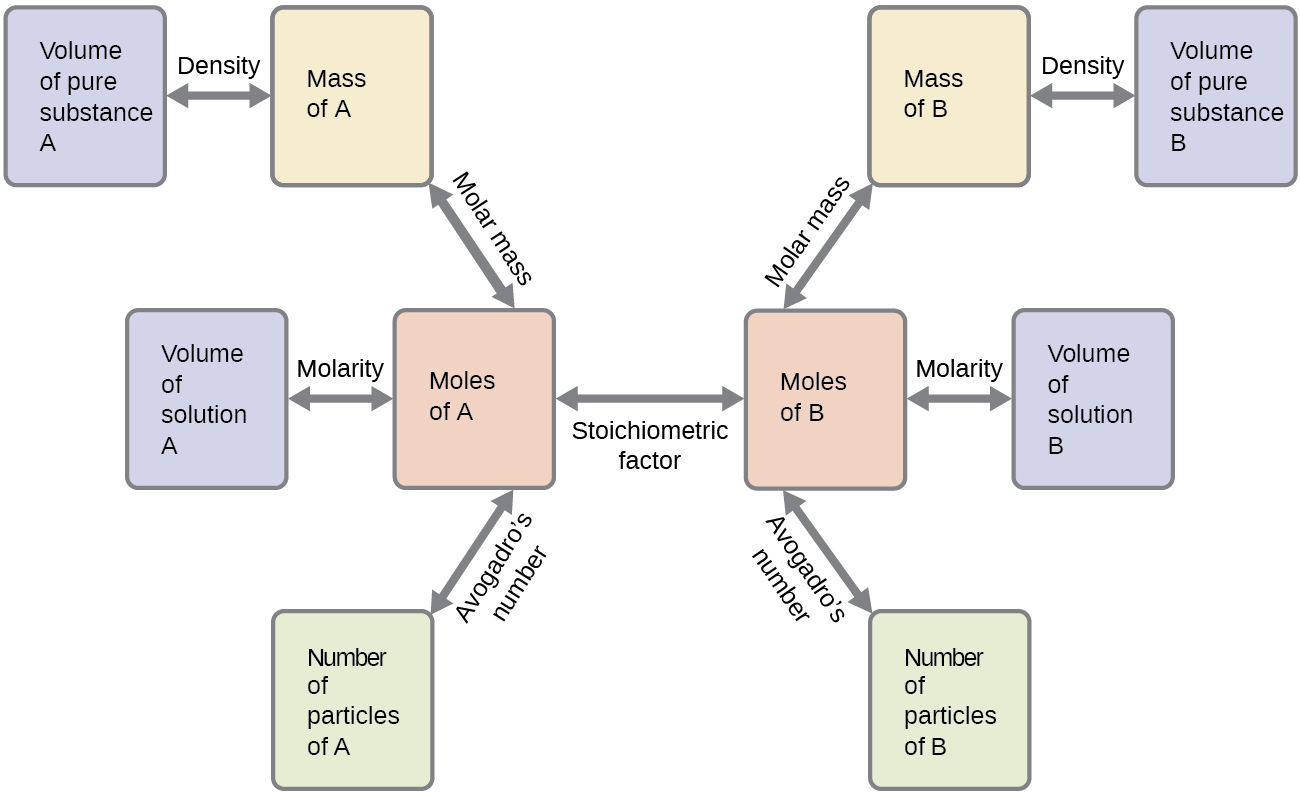
Stoichiometry Chemistry Activities
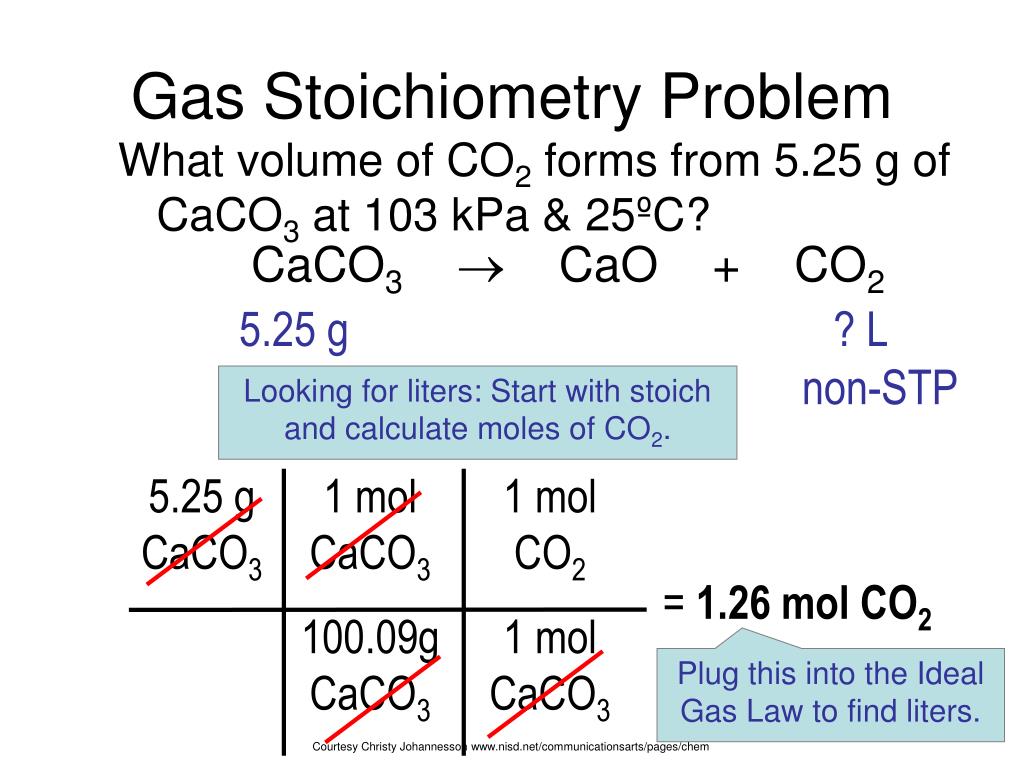
PPT Gas Stoichiometry PowerPoint Presentation, free download ID2956253
1 Kg = 1000 G = 2.20462 Lb.
Web This Stoichiometry Calculator Lets You Calculate The Relative Amounts Of Reactants And Products Involved In A Chemical Reaction.
Use The Mole Ratio To Find Moles Of Other Reactant.
Mgsio3 + H2Co3 = Mgco3 + H2Sio3.
Related Post: