Thermodynamic Favorability Chart
Thermodynamic Favorability Chart - Understanding the relative stability of molecules can be important for predicting relative reactivity of starting materials and the relative yields of potential products. The temperature conditions under which a process is thermodynamically favored (δg° < 0) can be predicted from the signs of δh° and δs°. When δh° > 0 and δs° < 0, the process is. Thermodynamic favorability refers to the likelihood of a reaction occurring based on its change in gibbs free energy. Web this form of the equation provides a useful link between these two essential thermodynamic properties, and it can be used to derive equilibrium constants from standard free energy changes and vice versa. Gibbs free energy and thermodynamic favorability. The standard free energy of formation of kcl ( s) at 298 k is − 409 kj/mol. Gibb's law of thermodynamics is a function that relates these three factors, which are important in determining the spontaneity of a chemical reaction. Web standard thermodynamic properties for selected substances. Explain how reaction conditions can determine the product ratio in a reaction in which there is competition between thermodynamic and kinetic control. Thermodynamic favorability refers to the likelihood of a reaction occurring based on its change in gibbs free energy. Web gibbs free energy and thermodynamic favorability (practice) | khan academy. If the t δ s term is less than δ. 2 kclo a 3 ( s) → 2 kcl ( s) + 3 o a 2 ( g) for the reaction. The temperature conditions under which a process is thermodynamically favored (δg° < 0) can be predicted from the signs of δh° and δs°. A pure element in its standard state has a standard free energy of formation of zero. Explain how reaction conditions can determine the product ratio in a reaction in which there is competition between thermodynamic and kinetic. Web both δh and δs are positive. Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention. Web standard thermodynamic properties for selected substances. The position of equilibrium and therefore the electrode potential depends on factors such as: Web the relationship shown in equation 13.7.7 allows us. The position of equilibrium and therefore the electrode potential depends on factors such as: Explain how reaction conditions can determine the product ratio in a reaction in which there is competition between thermodynamic and kinetic control. Web thermodynamically favourable means from high energy to low energy, or, put another way, from less stable to more stable. At low temperatures, δ. An instance of a thermodynamically favored process is the dissolution of nacl in water. Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention. Cell potential & thermodynamic favorability. Thermodynamic favorability means a reaction is spontaneous, or the reaction does not require energy to occur. This condition. Web increases in entropy (s) are also thermodynamically favorable. Web both δh and δs are positive. Determining the effect of temperature on thermodynamic favorability. Gibb's law of thermodynamics is a function that relates these three factors, which are important in determining the spontaneity of a chemical reaction. In the case of e. Increases in entropy (s) are also thermodynamically favorable. The position of equilibrium and therefore the electrode potential depends on factors such as: Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention. In this case, δ g will be negative if the magnitude of the t δ. Coli, the use of thermodynamically more favorable pathways may improve the efficiency of pathway protein use. If the change is negative, the reaction is thermodynamically favorable and will occur spontaneously. For a given process, the value of δg° can be calculated directly from. An instance of a thermodynamically favored process is the dissolution of nacl in water. Web thermodynamically favourable. Thermodynamic favorability refers to the likelihood of a reaction occurring based on its change in gibbs free energy. For any chemical reaction, the standard free energy change is the sum of. In the case of e. If the change is negative, the reaction is thermodynamically favorable and will occur spontaneously. Web gibbs free energy and thermodynamic favorability (practice) | khan. The general rule is that if the entropy of the universe (thermodynamic system plus its surroundings) is positive, then the reaction is thermodynamically favored. At low temperatures, δ go is positive and the reaction is thermodynamically favored. Web h 2 o ( l) → h 2 o ( g) δ h o = + 40.7 k j. In the case. For a given process, the value of δg° can be calculated directly from. If δs universe is negative, then the reaction is not thermodynamically favored. When δg° < 0, the process is thermodynamically favored. Web increases in entropy (s) are also thermodynamically favorable. Web the temperature conditions under which a process is thermodynamically favored (δg° is negative) can be predicted from the signs of δh° and δs°. Understanding the relative stability of molecules can be important for predicting relative reactivity of starting materials and the relative yields of potential products. Coli, the use of thermodynamically more favorable pathways may improve the efficiency of pathway protein use. Web gibbs free energy and thermodynamic favorability (practice) | khan academy. Web the thermodynamic favorability of reactions is controlled by changes in entropy and enthalpy, and the temperature at which the reaction takes place. This condition describes an endothermic process that involves an increase in system entropy. 2 kclo a 3 ( s) → 2 kcl ( s) + 3 o a 2 ( g) for the reaction represented above, the value of δ g ° 298 is − 226 kj/mol r x n. At low temperatures, δ go is positive and the reaction is thermodynamically favored. The general rule is that if the entropy of the universe (thermodynamic system plus its surroundings) is positive, then the reaction is thermodynamically favored. Web when δh° and δs° for a reaction have the same sign, the thermodynamic favorability of the reaction depends on temperature. When δh° < 0 and δs° > 0, the process is favored at all temperatures. Gibbs free energy and thermodynamic favorability.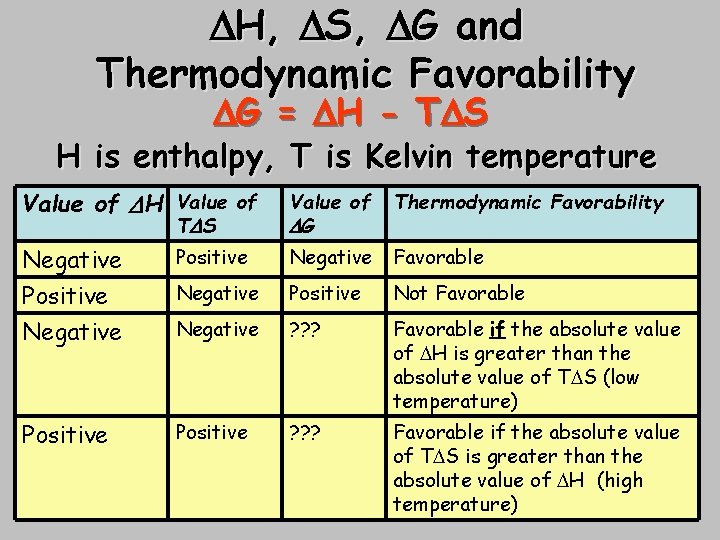
Enthalpy Entropy Free Energy and Spontaneity Thermodynamic Favorability

You should note that at low temperature, enthalpy is dominant, while at

Predicting thermodynamic favorability using energy diagrams YouTube

An illustrative example to show differences in ranking between

Thermodynamic favorability and pathway yield as evolutionary tradeoffs

Table of Thermodynamic Values

AP Chemistry Video 92 Thermodynamic Favorability and Equilibrium

Thermodynamic favorability and pathway yield as evolutionary tradeoffs

Thermodynamic Favorability

AP Chemistry 9.3 Gibbs Free Energy and Thermodynamic Favorability
Gibb's Law Of Thermodynamics Is A Function That Relates These Three Factors, Which Are Important In Determining The Spontaneity Of A Chemical Reaction.
Web Draw A Reaction Energy Diagram For A Reaction Which Can Result In Both A Thermodynamically Controlled Product And A Kinetically Controlled Product.
Web Thermodynamic Favorability Refers To The Intrinsic Propensity Of A Reaction To Occur Spontaneously Within A Closed System, Without Requiring Any External Intervention.
When Δh° > 0 And Δs° < 0, The Process Is.
Related Post: