Vapor Pressure Of Water Chart
Vapor Pressure Of Water Chart - That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Have you found yourself wondering: To understand that the relationship between pressure, enthalpy of vaporization, and temperature is given. Web the graph of the vapor pressure of water versus temperature in figure \(\pageindex{3}\) indicates that the vapor pressure of water is 68 kpa at about 90 °c. Nist chemistry webbook, srd 69. You can see the drinking duck in action in the video below: (1 atm = 101,325 pa) click on the icon to switch between si (kpa) and us customary (psi) units. Web with this vapor pressure calculator, we present to you two vapor pressure equations! Thus, at about 90 °c, the vapor pressure of water will equal the atmospheric. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. Thus, at about 90 °c, the vapor pressure of water will equal the atmospheric. Nist chemistry. 1 atmosphere pressure is 101.325 kpa. Vapor or saturation pressure depends on temperature. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas. The pressure scale (the vertical one) is measured in kilopascals (kpa). The drinking duck is a toy that many kids (and adults) enjoy playing with. If you're unsure what vapor pressure is, keep scrolling. The motion of the duck illustrates a physical principle called vapor pressure. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. 1 atmosphere pressure is 101.325 kpa. Web vapor pressure [a] or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. Note that when water vapor pressure equals atmospheric pressure, then the water molecules are free to jump into the gas state. Web with this vapor pressure calculator, we present to you two vapor pressure equations! Nist chemistry webbook, srd 69. Vapor or saturation pressure depends on. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. Web the vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid. How does a liquid change into a gas due to a change in pressure and temperature? Nist chemistry webbook, srd 69. Copyright © 2024 claude yoder. The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Web vapor pressure [a] or equilibrium vapor pressure is the pressure exerted by a vapor. At its freezing point (0 ° c), the vapor pressure of water is 4.6 torr. Web the following charts and table provide a comprehensive list of water vapor pressure at different temperatures under 1 atmospheric (atm) pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid). The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Web what causes this toy to move? Thus, at about 90 °c, the vapor pressure of water will equal the atmospheric. Web the graph of the vapor pressure of water versus temperature in figure \(\pageindex{3}\) indicates that the vapor pressure of. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapor pressure is directly proportional to temperature). Web with this vapor pressure calculator, we present to you two vapor pressure equations! As the vapor pressure changes, the liquid in the duck moves up and down, causing the duck to move. And what does this. Web the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Just type in the temperature, and the pressure will appear in no time — don't hesitate. Web the vapor pressure of water at room temperature (25 ° c) is 23.8 mm hg, 0.0313 atm, or 23.8 torr, or 3.17 kpa. You can see the drinking duck in action in the video below: Web what causes this toy to move? Copyright © 2024 claude yoder. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This chart shows that this trend is true for various substances with differing chemical properties. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c. Web with this vapor pressure calculator, we present to you two vapor pressure equations! Water vapour pressure table at different temperatures. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0. Vapor or saturation pressure depends on temperature. The pressure scale (the vertical one) is measured in kilopascals (kpa). Web the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Thus, at about 90 °c, the vapor pressure of water will equal the atmospheric.
Vapor Pressure Chart For Water
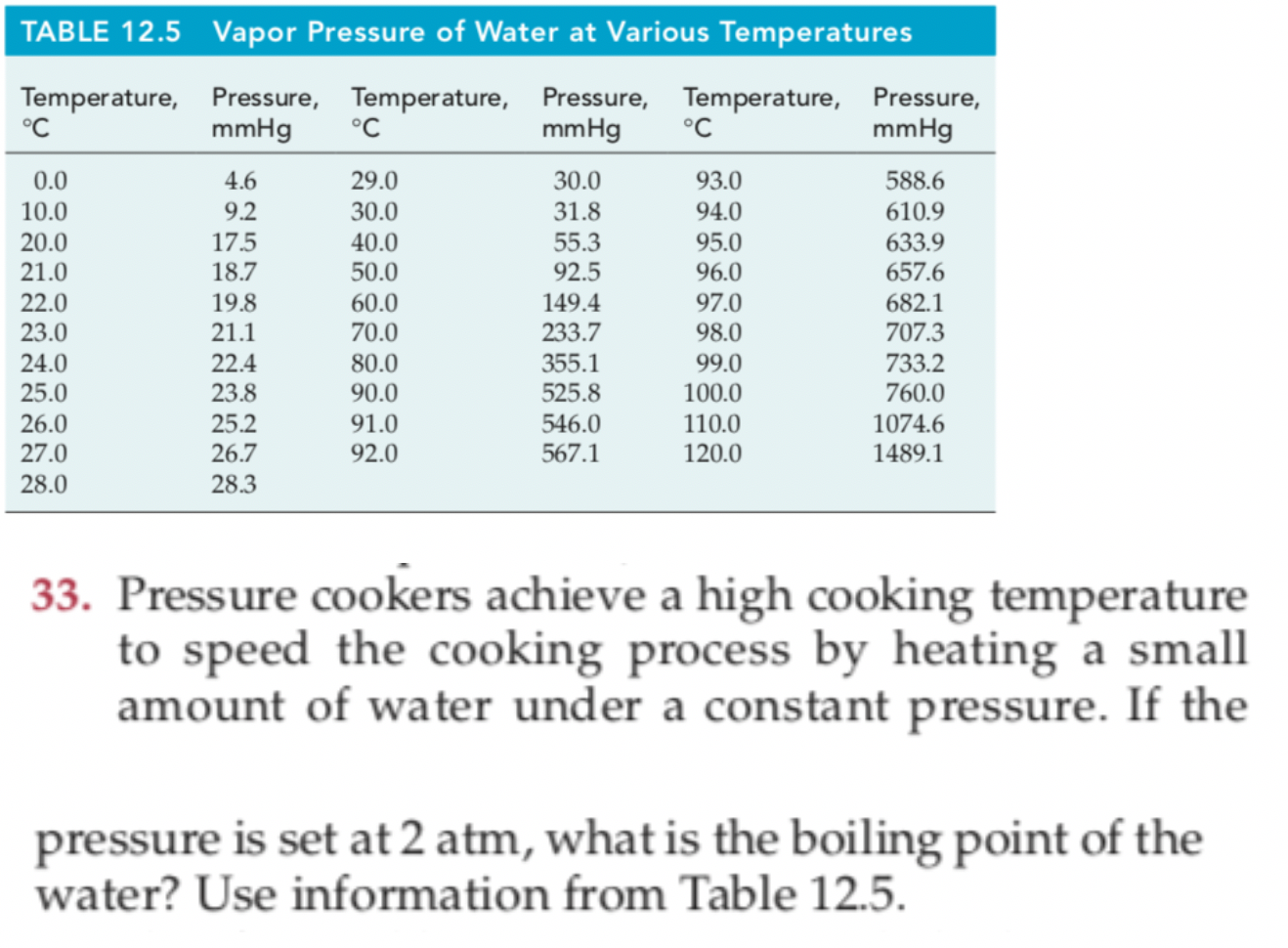
Vapor Pressure Chart For Water

Vapor Pressure Chart For Water
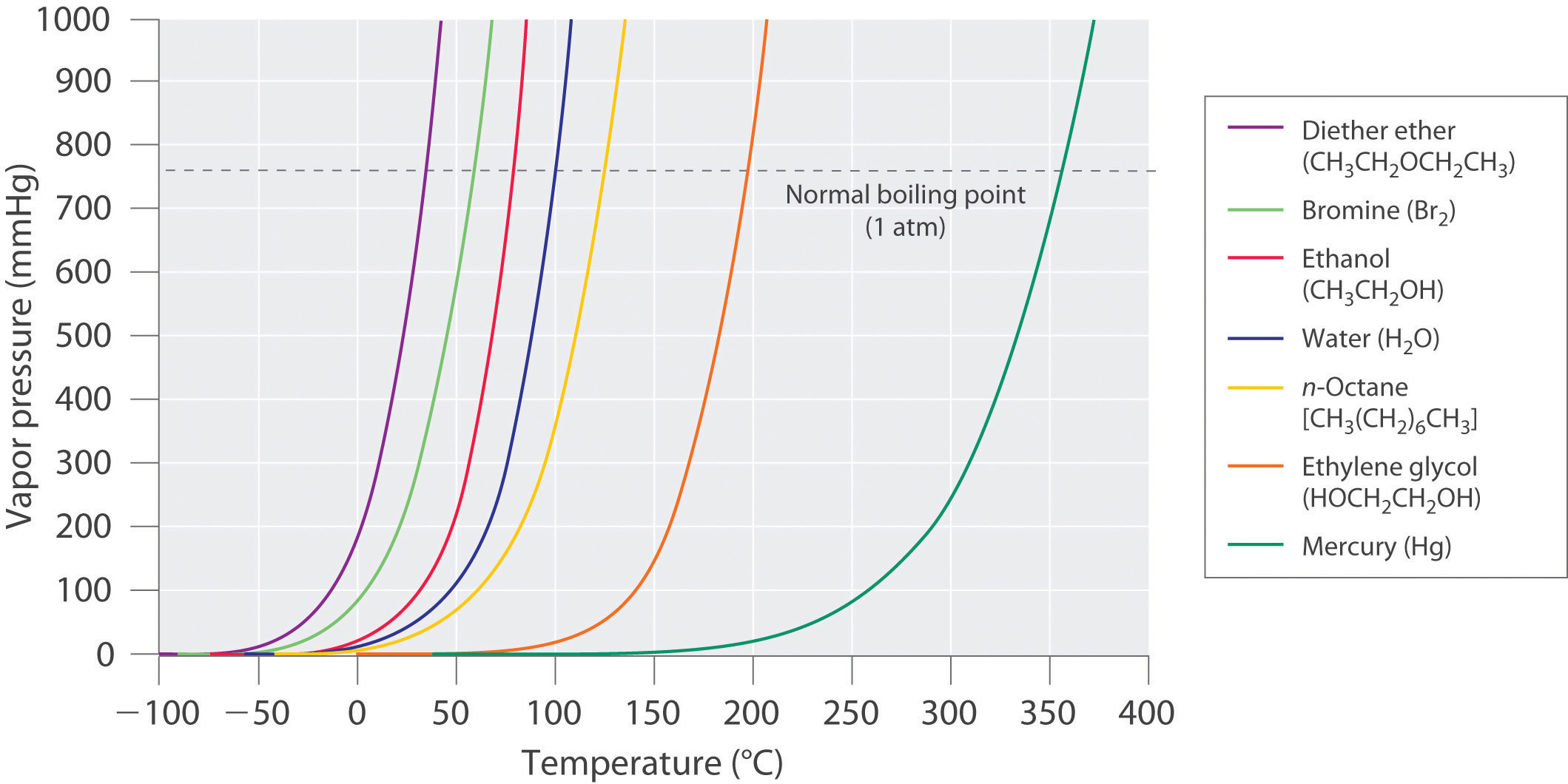
Chapter 11.4 Vapor Pressure Chemistry LibreTexts
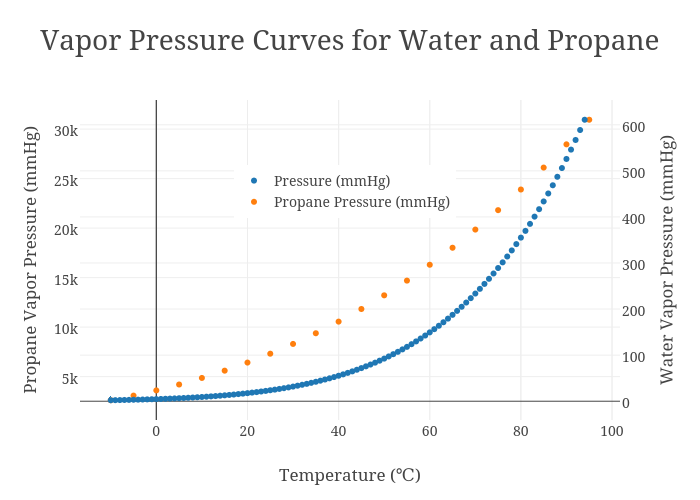
Vapor Pressure Curves for Water and Propane scatter chart made by
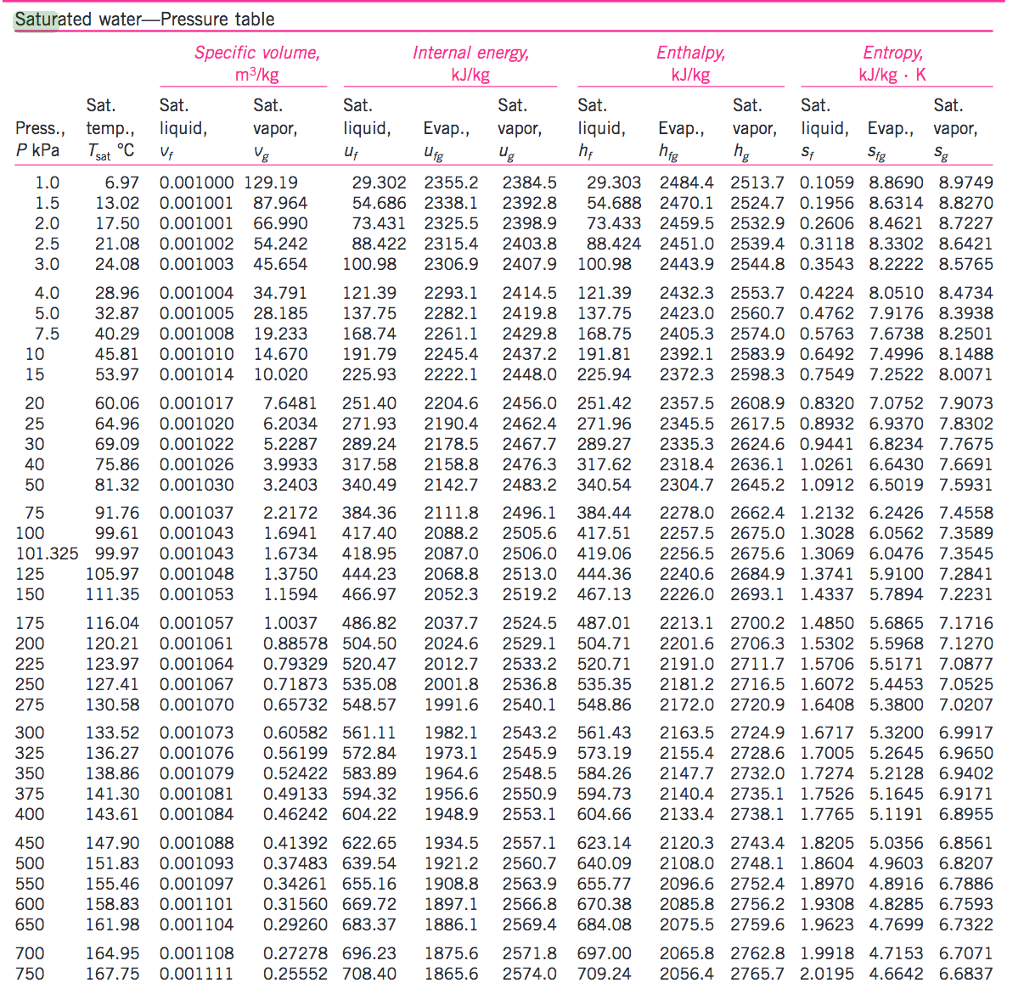
Vapour Pressure Of Water Chart

vapor pressure of water table

Conservation physics Fundamental microclimate concepts

Table Of Vapor Pressure Of Water A Guide To Understanding Its

Water Vapour Pressure Chart Bar
Web Vapor Pressure [A] Or Equilibrium Vapor Pressure Is The Pressure Exerted By A Vapor In Thermodynamic Equilibrium With Its Condensed Phases (Solid Or Liquid) At A Given Temperature In A Closed System.
That Is, The Pressure Of The Vapor Resulting From Evaporation Of A Liquid (Or Solid) Above A Sample Of The Liquid (Or Solid) In A Closed Container.
Web The Following Charts And Table Provide A Comprehensive List Of Water Vapor Pressure At Different Temperatures Under 1 Atmospheric (Atm) Pressure.
Vapor Pressure Is Directly Proportional To Temperature).
Related Post: