Chart Of Atomic Radius
Chart Of Atomic Radius - Atomic radius of some common elements. Smallest and largest atomic radius. The periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. The periodic table greatly assists in determining atomic radius and presents a number of trends. The editors of encyclopaedia britannica. Web atomic radius trends on periodic table (video) | khan academy. Pdf without crop marks | pdf with crop marks. Practice problems on atomic radius trends. Web atomic radii is useful for determining many aspects of chemistry such as various physical and chemical properties. Explain why the atomic radius of hydrogen is so much smaller than the atomic radius of potassium. Want to join the conversation? You should consult reference 1 for full details, but it is not light reading for most people. Web define “atomic radius.” what are the units of measurement for atomic radius? The periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. The editors of encyclopaedia britannica. Below mentioned radii are the van der waals radius in picometer (pm)). 1 å = 1 × 10−10 m = 100 pm. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Click on periodic table push button. Practice problems. Web atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its relationship to other atomic properties such as nuclear charge and shielding. Web table of content. Web explore how atomic radius changes with atomic number in the periodic table of. Atomic radii can be obtained from quantum mechanical calculations or. Web the values given here for atomic radius are calculated values using methods outlined in reference 1. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Below mentioned radii are the van der waals radius in picometer (pm)). Web the. Below mentioned radii are the van der waals radius in picometer (pm)). Click on radio button atomic radius. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Web the atomic radius may refer to the ionic radius, covalent radius, metallic radius, or van der waals. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. Types of atomic radius with respect to the types of bond. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. As shown in the graph below, the atomic radius is largest at the first element in each. Smallest and largest atomic radius. Web the atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus. Web interactive periodic table showing names, electrons, and oxidation states. Web atomic radius of all the elements are mentioned in the chart below. Web the atomic radius. Want to join the conversation? Web atomic radius of all the elements are mentioned in the chart below. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. The way the atomic radius varies with increasing atomic number can be explained by the arrangement of electrons in shells of fixed capacity. Atomic radius of an atom. The covalent radius of an atom is the radius of an atom under the covalent bond with another atom (s) of a similar element. Click on radio button atomic radius. How atomic radius is defined, and trends across a period and down a group. The periodic table greatly assists in determining atomic radius and presents a number of trends. Atomic. You should consult reference 1 for full details, but it is not light reading for most people. Web interactive periodic table showing names, electrons, and oxidation states. The general trend is that atomic sizes increase as one moves downwards in the periodic table of the elements, as electrons fill outer electron shells. Covalent radius can be calculated by measuring the. Explain why the atomic radius of hydrogen is so much smaller than the atomic radius of potassium. Covalent radius can be calculated by measuring the distance between the two nucleus of two atoms in covalent compound. Web atomic radii is useful for determining many aspects of chemistry such as various physical and chemical properties. The periodic table greatly assists in determining atomic radius and presents a number of trends. The covalent radius of an atom is the radius of an atom under the covalent bond with another atom (s) of a similar element. 1 å = 1 × 10−10 m = 100 pm. Francium has the largest atomic size on the periodic table, and helium has the smallest atomic size. How does the atomic radius of different elements change across a period? The general trend is that atomic sizes increase as one moves downwards in the periodic table of the elements, as electrons fill outer electron shells. Based on the type of bond, atomic radius is divided into three types as follows: (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Web atomic radius trends on periodic table (video) | khan academy. Web table of content. Web the trend on a graph. The way the atomic radius varies with increasing atomic number can be explained by the arrangement of electrons in shells of fixed capacity. The periodic table contains nist’s latest critically evaluated data for atomic properties of the elements..png)
Periodic Table Of The Elements Atomic Radius vrogue.co
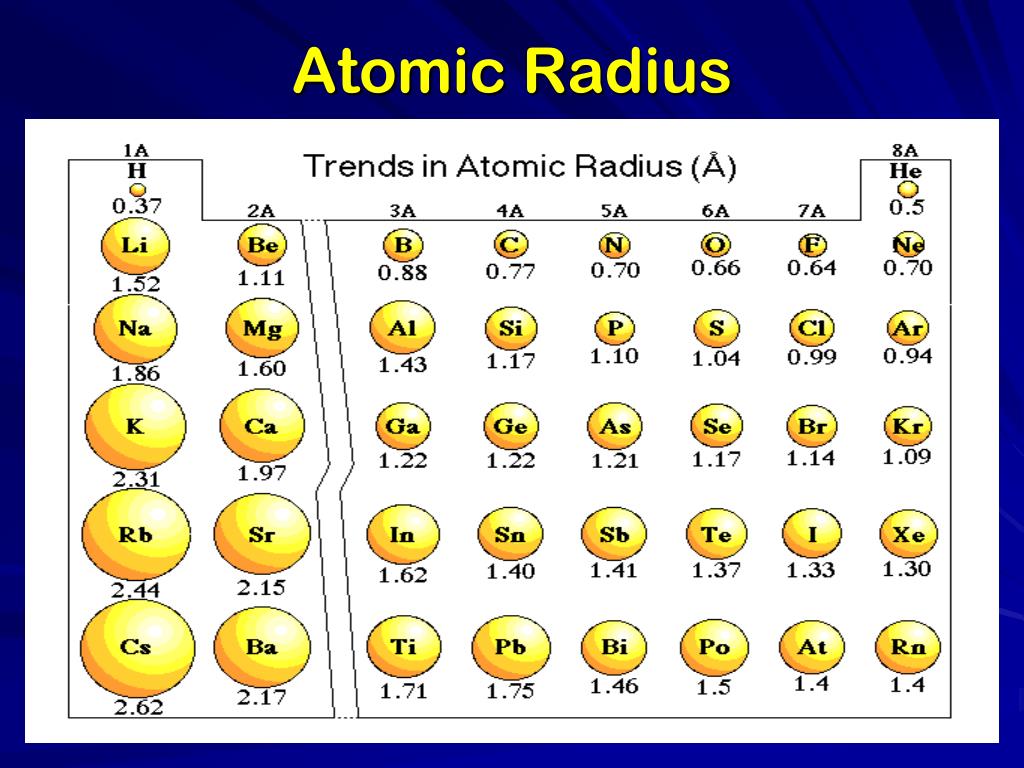
PPT Periodic Table PowerPoint Presentation, free download ID2617702

Atomic Sizes and Radii Charts for Chemistry
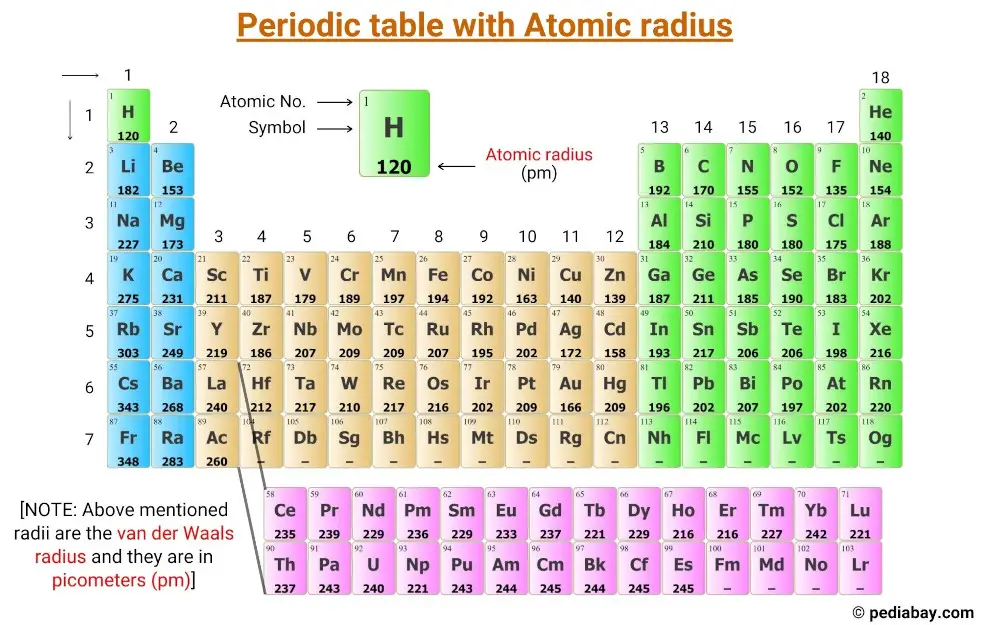
Atomic Radius of Elements (With Periodic table Chart) Pediabay

Atomic Radius of Elements
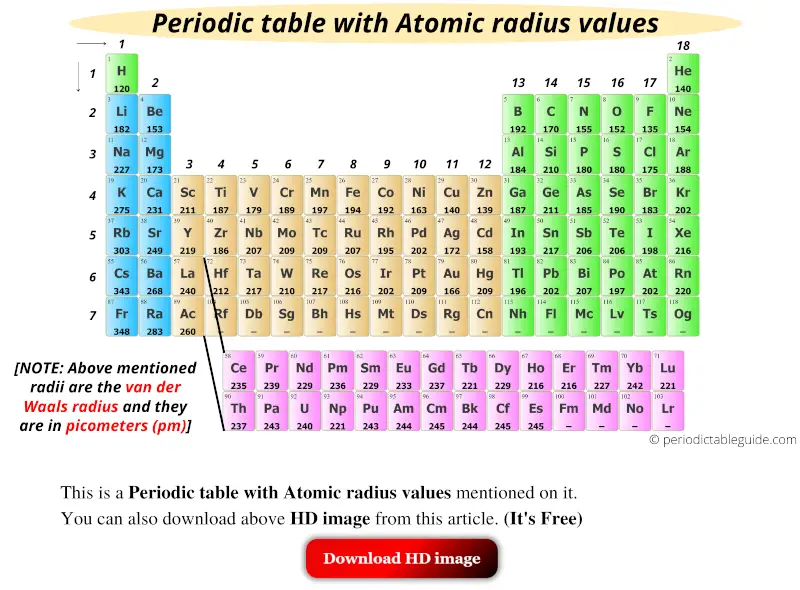
Get the Periodic table with Atomic radius values (Img+Chart)
Periodic Behavior Presentation Chemistry
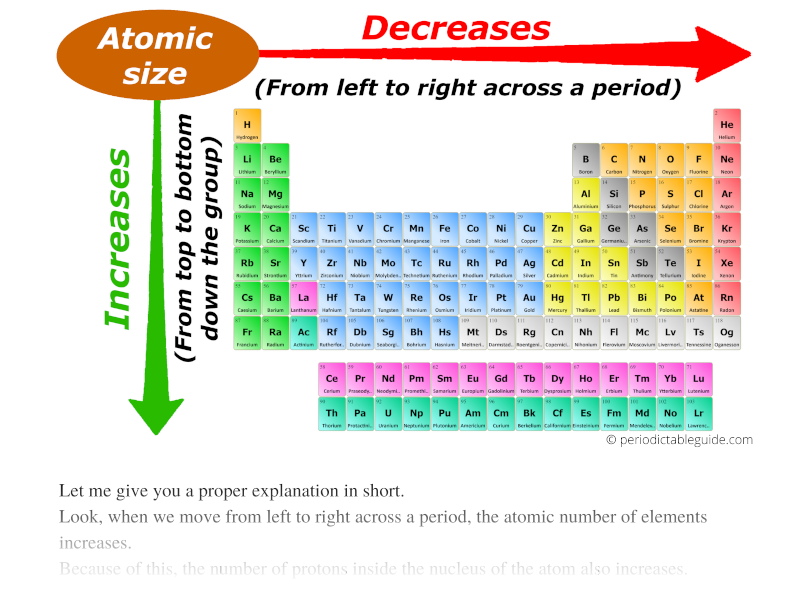
Atomic radius chart mindsstorm

Atomic Radius of Elements The Periodic Table

Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table
Web The Atomic Radius May Refer To The Ionic Radius, Covalent Radius, Metallic Radius, Or Van Der Waals Radius.
Atomic Radius Of Some Common Elements.
Atomic Radius Periodic Table Trends.
Click On Radio Button Atomic Radius.
Related Post:
.PNG)