Covalent Radius Chart
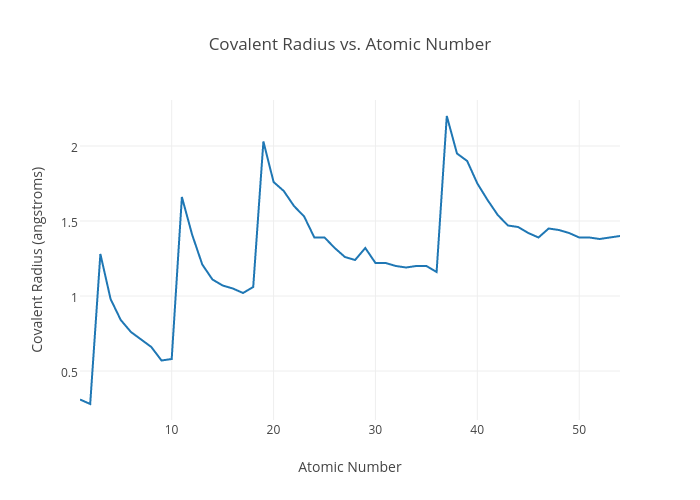
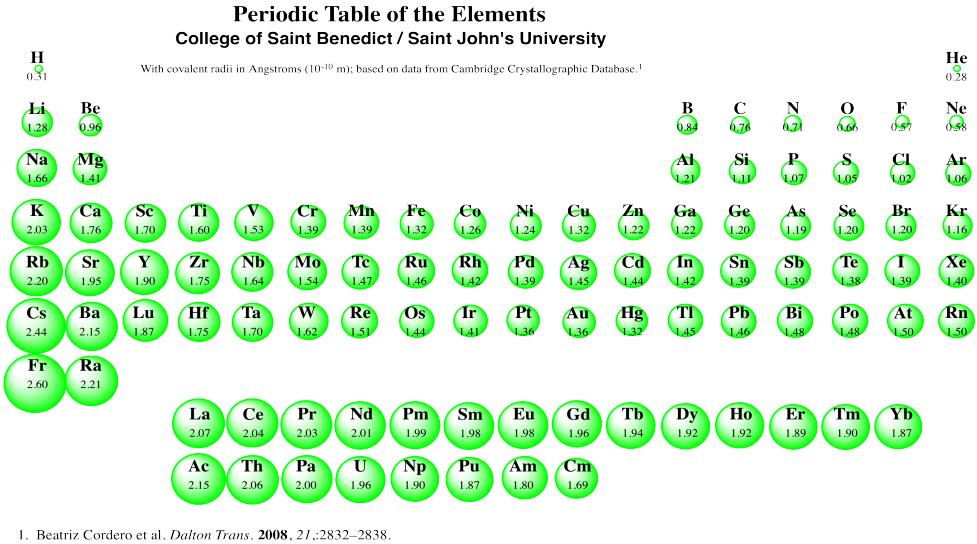
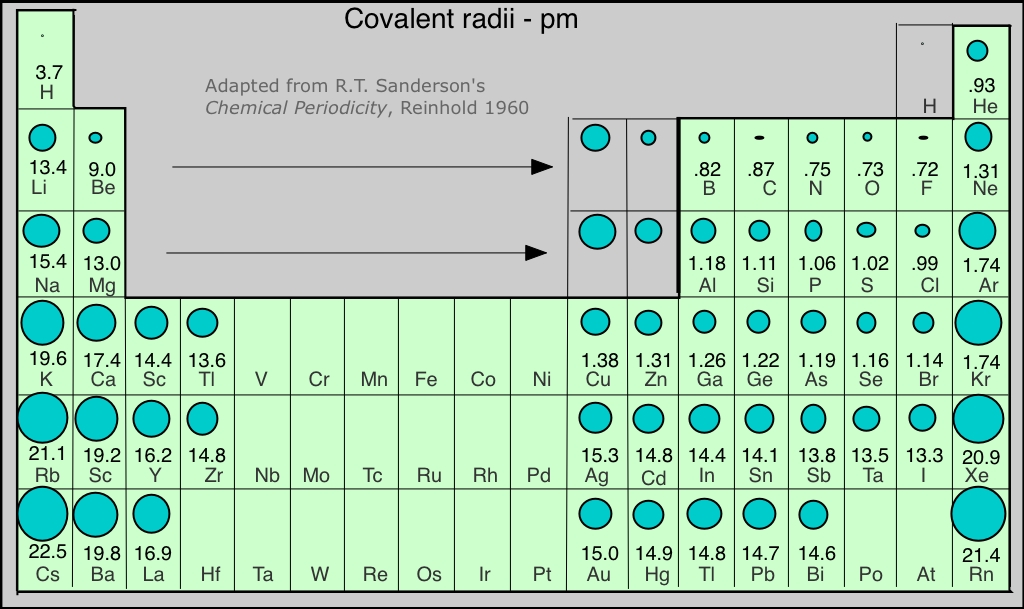
Covalent Radius Chart - In principle, the sum of the two covalent radii should equal the covalent bond length between two atoms, r (ab) = r (a) + r (b). Web the covalent radius, r cov, is a measure of the size of an atom that forms part of one covalent bond. 292 (covalent radii for nonmetals); It is measured either in picometres (pm) or ångströms (å), with 1 å = 100 pm. Point to the graph to see details, or click for full data on that element. Web covalent radius is expressed in terms of picometers (pm) or angstroms (å). Web covalent radius when a covalent bond is present between two atoms, the covalent radius can be determined. Web the covalent radius of an atom is the radius of an atom under the covalent bond with another atom (s) of a similar element. Click here to buy a book, photographic periodic table poster, card deck, or 3d print based on the images you see here! Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is defined as half the distance between the nuclei of two adjacent atoms in a metallic element. Web covalent radius of the elements. Web the values of table 3 show the covalent radii from the two recent original determinations by cordero et al. When two atoms of the same element are covalently bonded, the radius of each atom will be half the distance between. Usually, you see covalent radius in units of picometers (pm) or angstroms (å),. It is measured either in picometres (pm) or ångströms (å), with 1 å = 100 pm. [1], and pyykkö and atsumi [2]. Wells, structural inorganic chemistry, 5th ed., clarendon press, oxford, 1984, p. In the case of a simple diatomic molecule, the covalent radius is half the distance between the nuclei. Web the covalent radius of an atom is the. Point to the graph to see details, or click for full data on that element. In principle, the sum of the two covalent radii should equal the covalent bond length between two atoms, r (ab) = r (a) + r (b). Web covalent radii are in parentheses. Web for facts, physical properties, chemical properties, structure and atomic properties of the. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. When two atoms of the same element are covalently bonded, the radius of each atom will be half the distance between. Web covalent radius is expressed in terms of picometers (pm) or angstroms (å). In principle, the sum of the two covalent radii should equal the. It is usually measured either in picometres (pm) or angstroms (å), with 1 å = 100 pm. Wells, structural inorganic chemistry, 5th ed., clarendon press, oxford, 1984, p. Web the covalent radius of an atom is the radius of an atom under the covalent bond with another atom (s) of a similar element. There are several methods that can be. Web it is often denoted by a0 and is approximately 53 pm. It is measured either in picometres (pm) or ångströms (å), with 1 å = 100 pm. Wells, structural inorganic chemistry, 5th ed., clarendon press, oxford, 1984, p. Don't let static charges disrupt your weighing accuracy. Web covalent radius of the elements. When two atoms of the same element are covalently bonded, the radius of each atom will be half the distance between. Web the covalent radius, r cov, is a measure of the size of an atom that forms part of one covalent bond. Web covalent radius of the elements. [1], and pyykkö and atsumi [2]. Charts are also compiled for. Click here to buy a book, photographic periodic table poster, card deck, or 3d print based on the images you see here! In theory, the sum of two covalent radii should equal the covalent bond length between two atoms, but in practice the length of the bond depends on the chemical environment. In the case of a simple diatomic molecule,. It is usually measured either in picometres (pm) or angstroms (å), with 1 å = 100 pm. Don't let static charges disrupt your weighing accuracy. Below mentioned radii are the van der waals radius in picometer (pm)). It is measured either in picometres (pm) or ångströms (å), with 1 å = 100 pm. [1], and pyykkö and atsumi [2]. Web the covalent radius of an atom is the radius of an atom under the covalent bond with another atom (s) of a similar element. Web the covalent radius, r cov, is a measure of the size of an atom that forms part of one covalent bond. In principle, the sum of the two covalent radii should equal the covalent. An effective radius assigned to an atom in a covalent compound. [1], and pyykkö and atsumi [2]. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Web complete and detailed technical data about the element $$$elementname$$$ in the periodic table. The radius of an atom is derived from the bond lengths within nonpolar molecules; Web covalent radii are in parentheses. Web covalent radius is expressed in terms of picometers (pm) or angstroms (å). Web atomic radius of all the elements are mentioned in the chart below. Web the covalent radius is defined as half the distance between two atoms of the same element that are covalently bonded. Below mentioned radii are the van der waals radius in picometer (pm)). Web for facts, physical properties, chemical properties, structure and atomic properties of the specific element, click on the element symbol in the below periodic table. In principle, the sum of the two covalent radii should equal the covalent bond length between two atoms, r (ab) = r (a) + r (b). It depends on the type of bond (single, double,.) and the electronegativity of the bonding partners. Click here to buy a book, photographic periodic table poster, card deck, or 3d print based on the images you see here! Wells, structural inorganic chemistry, 5th ed., clarendon press, oxford, 1984, p. Don't let static charges disrupt your weighing accuracy.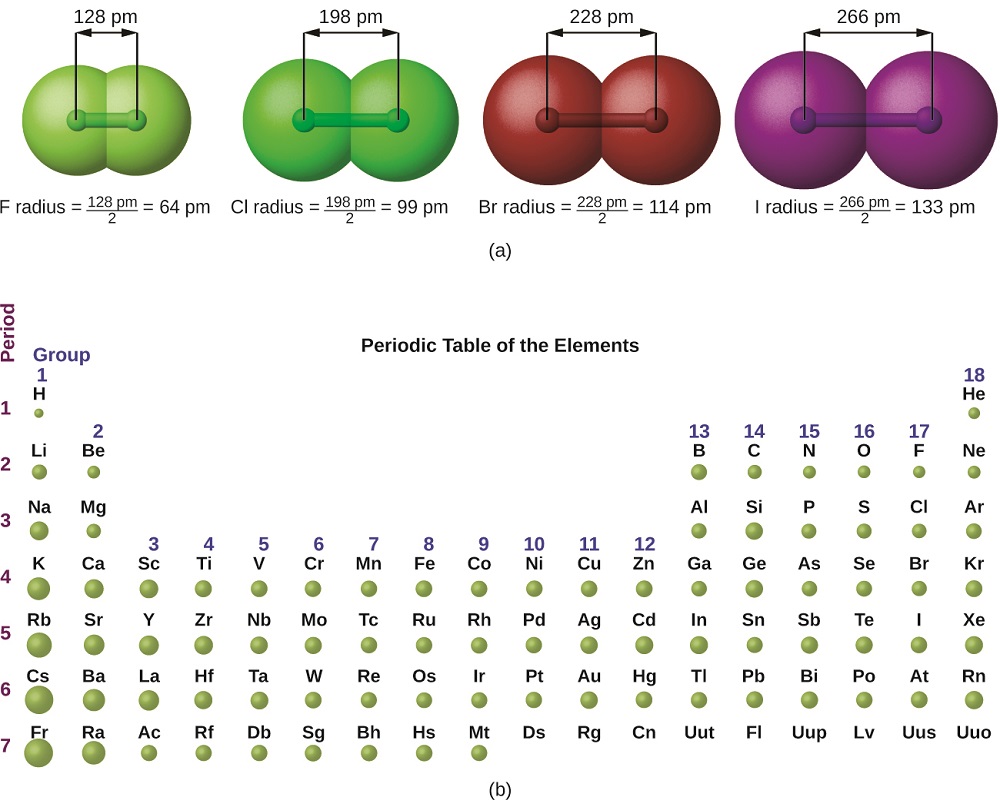
7.4 Electron Configurations, Valence Electrons, and the Periodic Table
Covalent Radius Chart A Visual Reference of Charts Chart Master
Covalent Radius
2.10 Periodic Properties of the Elements Chemistry LibreTexts
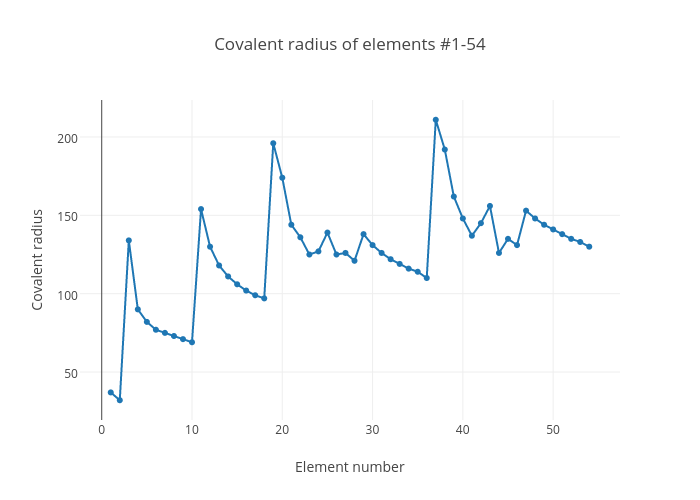
Covalent Radius Chart A Visual Reference of Charts Chart Master

Covalent Radius Definition and Trend
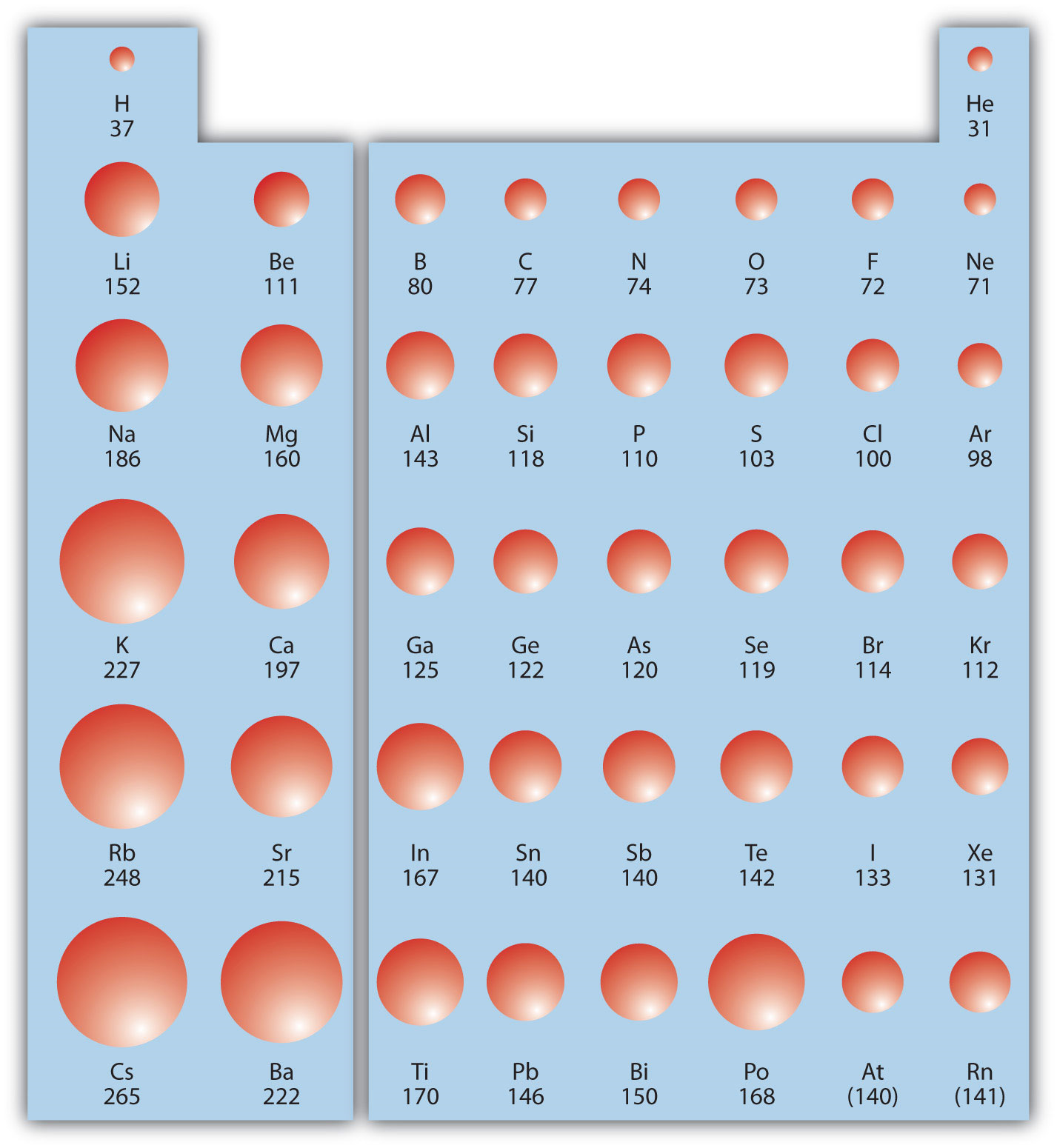
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic

What is the Atomic Radius? EnthuZiastic
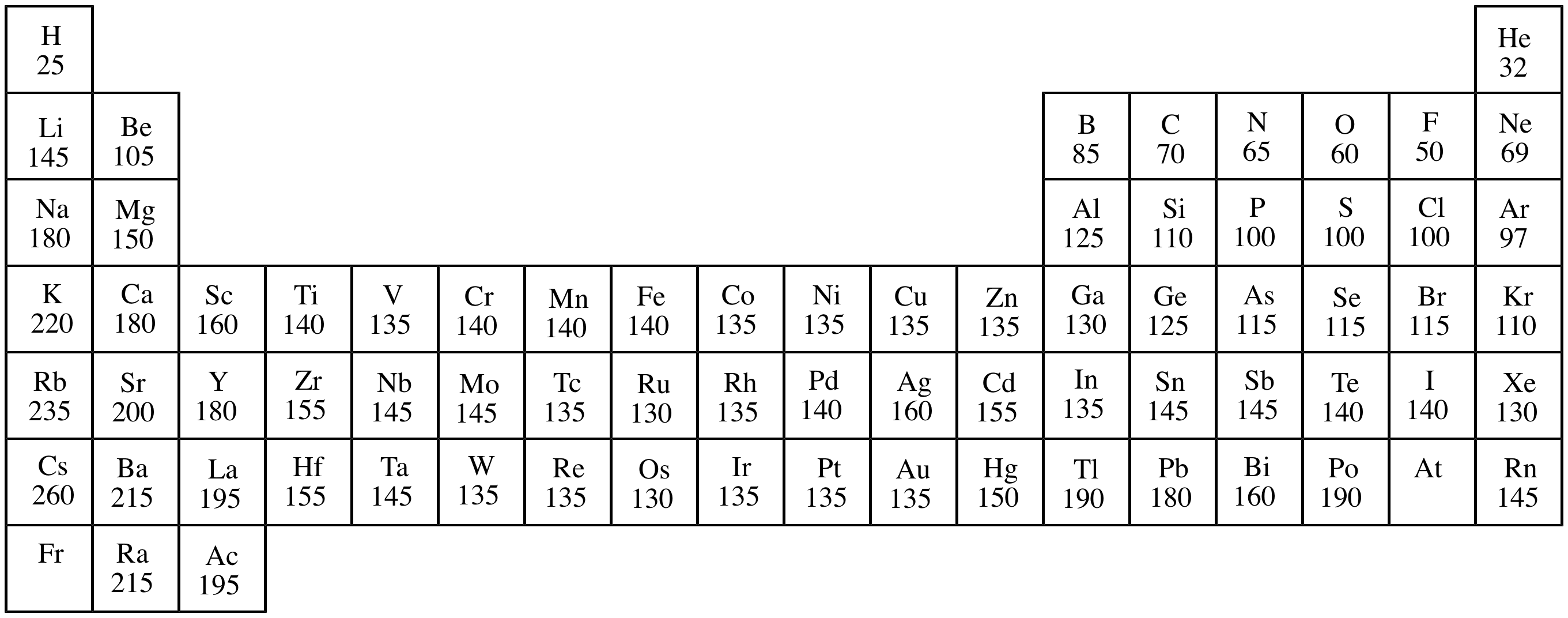
Periodic Table Atomic Radius Elcho Table

Smallest to largest atomic radius fleetlokasin
There Are Several Methods That Can Be Used To Determine Radii Of Atoms And Ions:
Web The Covalent Radius Of An Atom Is The Radius Of An Atom Under The Covalent Bond With Another Atom (S) Of A Similar Element.
It Is Measured Either In Picometres (Pm) Or Ångströms (Å), With 1 Å = 100 Pm.
For Example The Average Covalent Radius For Hydrogen Is 31 Pm And The Average Neon Covalent Radius Is 58 Pm.
Related Post: